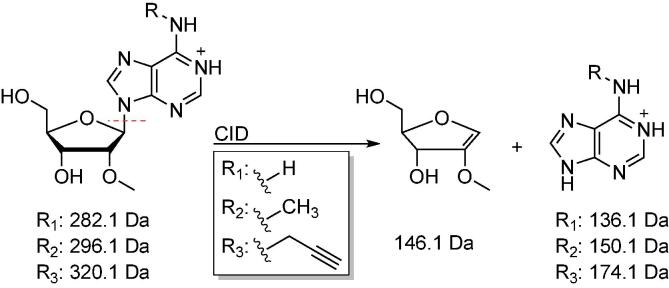
Scheme 3.
Representation of typical fragmentation patterns for nucleosides shown for Am, m6Am, N6pAm. Nucleosides typically fragment at their N-glycosidic bond, resulting in loss of ribose (-132 Da) or 2′-O-methyl ribose (146.1 Da, shown here), depending on the nucleoside analyzed. Increased collision energies typically result in further fragmentation of the nucleobase.