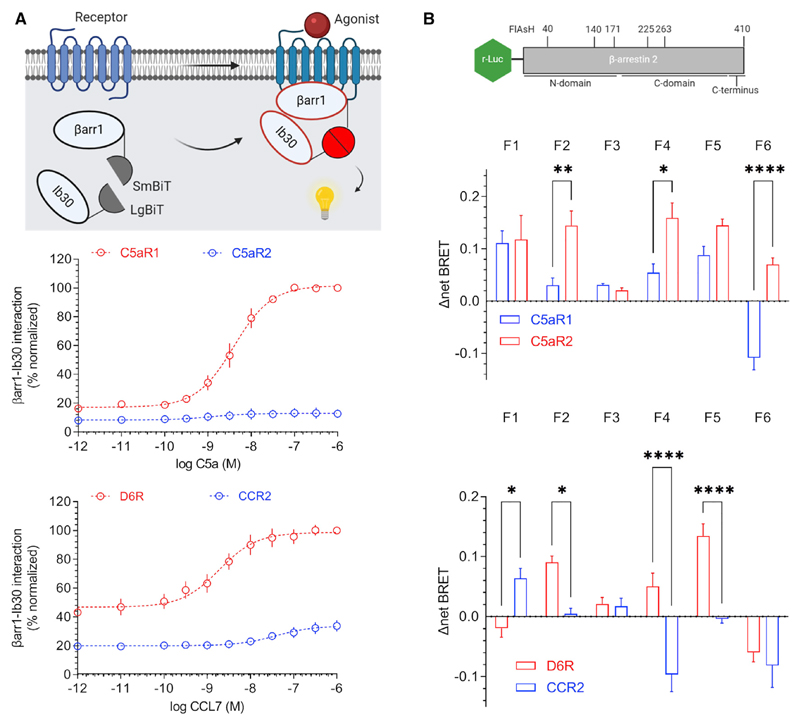
Figure 4. D6R and C5aR2 impart distinct conformations in βarrs.
(A) Intrabody30-based conformational sensor developed in the NanoBiT format (top panel) reveals distinct conformations of βarr1 for C5aR1-C5aR2 and D6R-CCR2 pairs. HEK293 cells expressing the indicated receptor, LgBiT-Ib30, and SmBiT-βarr1 were stimulated with various concentrations of agonists, and the luminescence signal was monitored. Data (mean ± SEM) from four independent experiments, normalized with respect to the maximal response (at 1 μM agonist concentration) in the receptor pairs, i.e., C5aR1 in the C5aR1-C5aR2 pair and D6R in the D6R-CCR2 pair (treated as 100%), are presented.
(B) Intramolecular BRET sensors of βarr2 reveal distinct conformational signatures in βarr2 for C5aR1 versus C5aR2 and D6R versus CCR2. The top panel shows the schematic of sensors where the N terminus of βarr2 harbors r-Luc (Renilla luciferase) as the BRET donor, whereas the FlAsH motif (as BRET acceptor) is encoded in various positions in βarr2. HEK293 cells expressing the indicated receptor and sensor constructs were labeled with the FlAsH reagent followed by agonist stimulation and measurement of the BRET signal. Data (mean ± SEM) from four independent experiments are presented (two-way ANOVA; *p < 0.05, **p < 0.01, ****p < 0.0001).