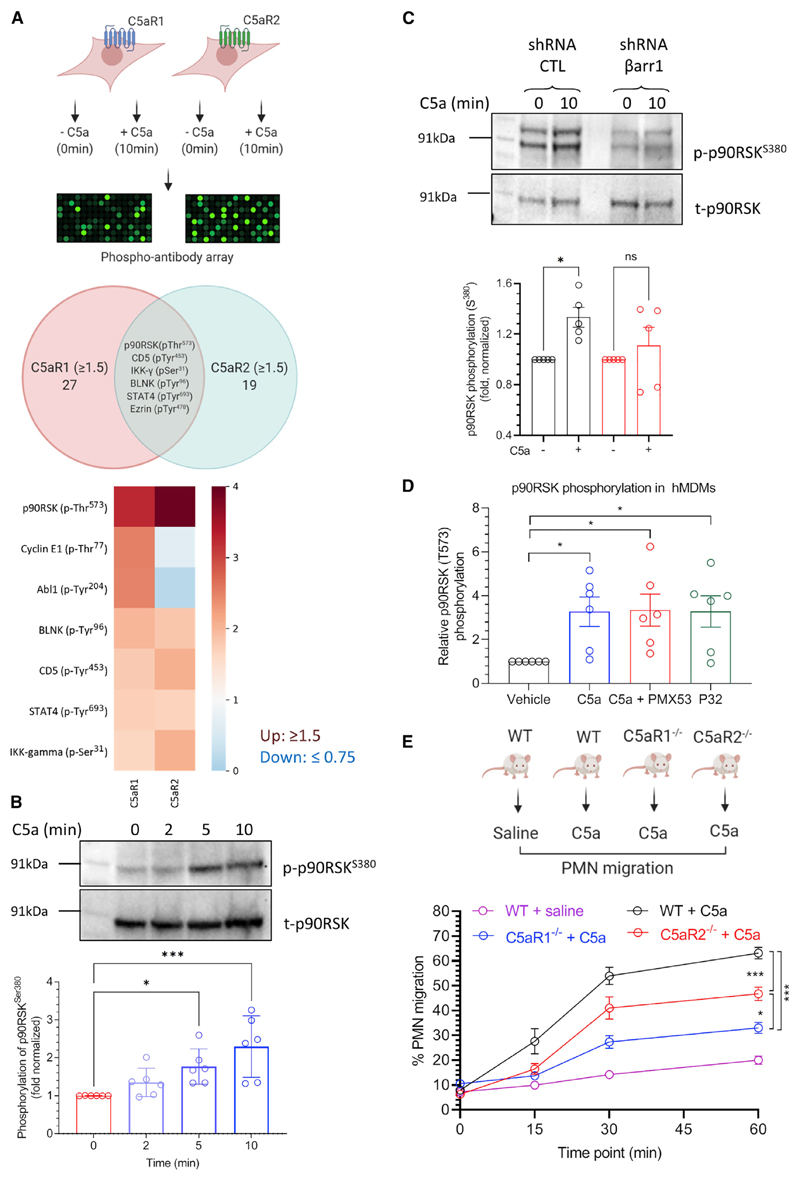
Figure 7. Signaling and functional outcomes of C5aR2 activation.
(A) Phospho-antibody array on HEK293 cells expressing C5aR1 or C5aR2 reveals phosphorylation/dephosphorylation of a few common and several distinct cellular proteins. Of these proteins, C5a stimulation of C5aR2-expressing HEK293 cells exhibits robust enhancement of p90RSK phosphorylation, which is also common to C5aR1.
(B) C5a-induced phosphorylation of p90RSKSer380 in C5aR2-expressing HEK293 cells is validated using western blotting. A representative blot and densitometry-based quantification (mean ± SEM) from six independent experiments are presented (*p < 0.05, ***p < 0.001; one-way ANOVA).
(C) C5a-induced phosphorylation of p90RSKSer380 is attenuated upon βarr1 knockdown in HEK293 cells expressing C5aR2. A representative blot and densitometry-based quantification (mean ± SEM) from five independent experiments are presented (*p < 0.05; one-way ANOVA).
(D) Stimulation of human-macrophage-derived monocytes (hMDMs) with either C5a or P32 (a C5aR2-selective agonist) results in phosphorylation of p90RSKThr573. Importantly, PMX53, a C5aR1-selective antagonist, does not block p90RSKThr573 phosphorylation, suggesting that it is mediated by C5aR2.
(E) A significant component of C5a-induced polymorphonuclear leukocyte (PMN) mobilization depends on C5aR2. Wild-type (WT) and C5aR1−/− and C5aR2−/− mice on a C57BL/6J genetic background (n = 5–15) were intravenously administered with recombinant mouse C5a (50 μg kg−1). Blood collected from the tail tip was smeared onto a slide, followed by staining and counting of white blood cells, with the proportion of PMNs calculated. Data are presented as mean ± SEM (p < 0.05, ***p < 0.001; one- and two-way ANOVA). See also Figure S7 and Table S1.