Figure 3.
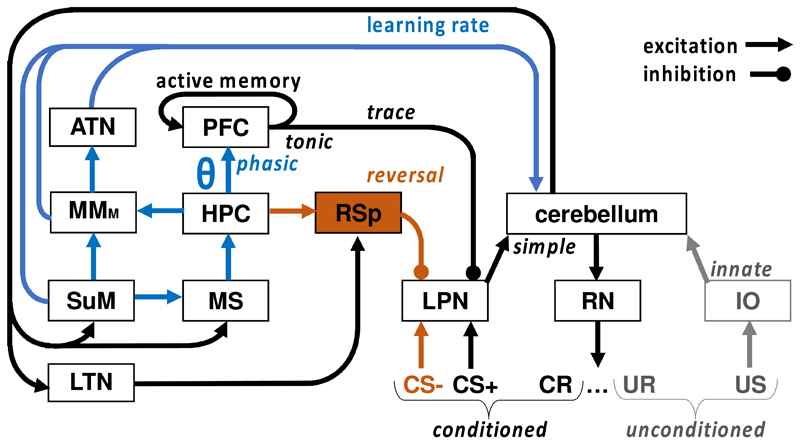
The role of the hippocampus (HPC) in eyeblink conditioning in mammals, based on [134-138]. HPC cells show firing patterns – triggered by the conditional (CS) but not unconditional (US) stimulus – that arise in training, progress during conditioning, and often model the conditioned eyeblink response (CR). Note that this combination of stimulus control with response-related firing implies that the hippocampal circuit is processing complex goal information rather than simple stimuli or actions. Hippocampal lesions do not affect simple, or delayed, or discriminative (CS+/CS-) conditioning. However hippocampal lesions affect both trace conditioning and discrimination reversal learning. Trace conditioning is mediated via output from delay-line activity from prefrontal cortex to lateral pontine nuclei (LPN) that inhibits activation of the eyeblink by the CS+ (in this case there is no CS-). Reversal is mediated via output from the retrosplenial cortex (RSp) that inhibits activation of the eyeblink by the CS- (which was the CS+ until reversal was started). Hippocampal theta-related output from HPC via the supramammillary nucleus, medial mammillary nucleus (MMM), and anterior thalamus (ATN) via pontine nuclei [139], impacts rate of learning [138]. Note that, in humans, “comparable delay and trace activation was measured in the cerebellum, whereas greater hippocampal activity was detected during trace compared with delay conditioning” [140] and there is good evidence for involvement of such cerebellar circuits in working memory generally [99].