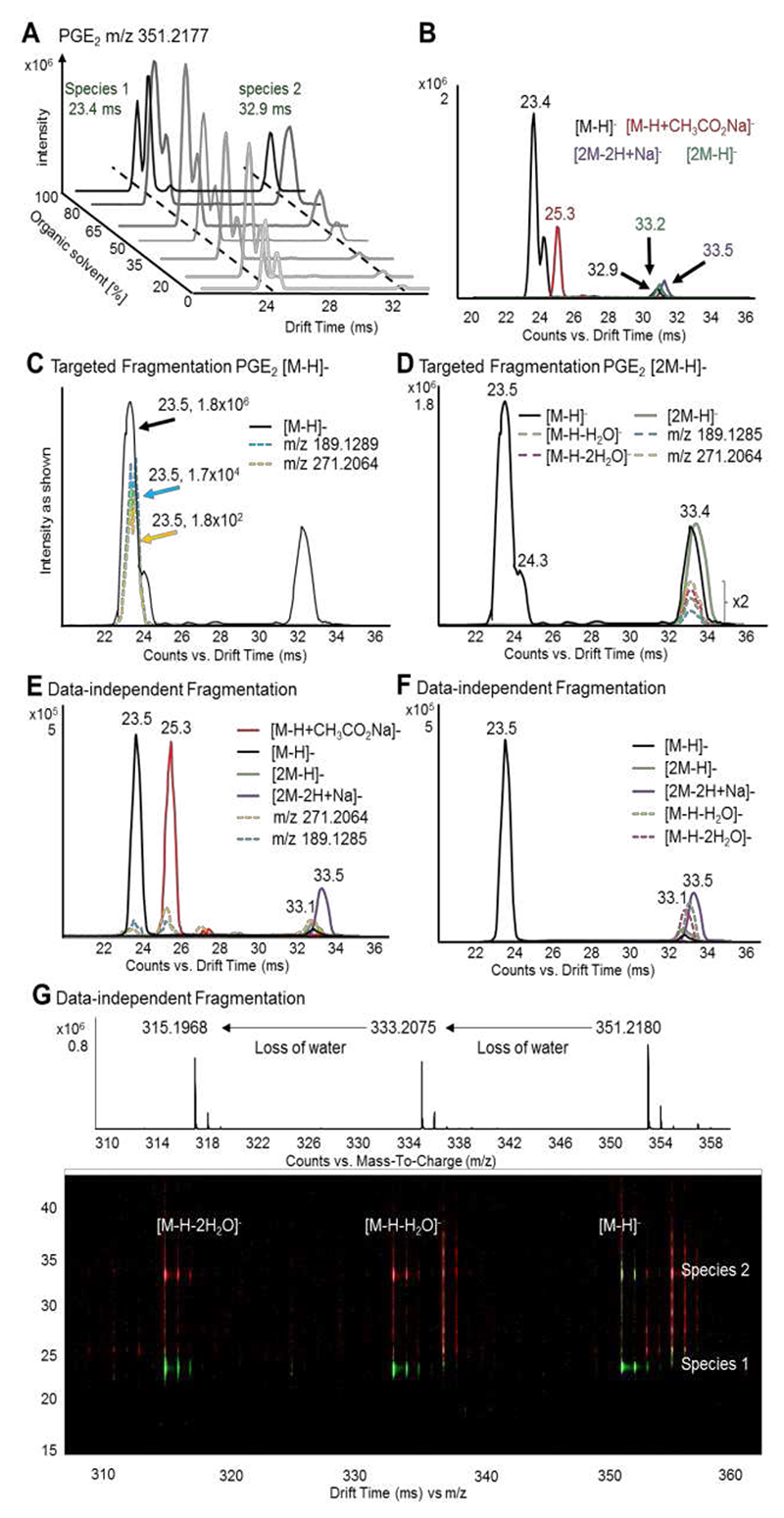
Figure 1. DTIM allows separation of adducts and alignment of pre-cursor and product ions following fragmentation.
(A) PGE2 standard was analyzed by flow injection DTIM-MS using varying concentrations of acetonitrile:methanol. The intensity of the two most abundant [M-H]- conformer is plotted versus the DT and the concentration of organic solvent (average DTs in green). (B) Drift spectra for the conformers [M-H]- (black), [M-H+CH3CO2Na]- (red), [2M-H]- (green) and [2M-2H+Na]- (purple). (C) Targeted DTIM-MS/MS analysis of the [M-H]- species (black) enables drift-time alignment with the diagnostic product ions m/z 271.2064 (yellow) and m/z 189.1285 (light blue). (D) Similarly, fragmentation of [2M-H]- (green) results in product ions m/z 271.2064, m/z 189.1285, as well as water loss products [M-H-H2O]- (dashed green) and [M-H-2H2O]- (dashed pink) co-drifting with their precursor at 33.4 ms. Product ion peaks are magnified by factor 2. Data-independent acquisition (DIA) gives similar results, with product ions m/z 271.2064 and m/z 189.1285 as well as species [M-H-H2O]- and [M-H-2H2O]- time-aligning to their precursor ions [M-H]-, [2M-H]-, [M-H+CH3CO2Na]- (red), and [2M-2H+Na]- (purple). (E, F). The driftogram obtained from DIA (G) highlights how water-loss products deriving from both species 1 at 23.4 ms and 2 at 32.9 ms can be products (red, high fragmentation) and precursors (green, low fragmentation).