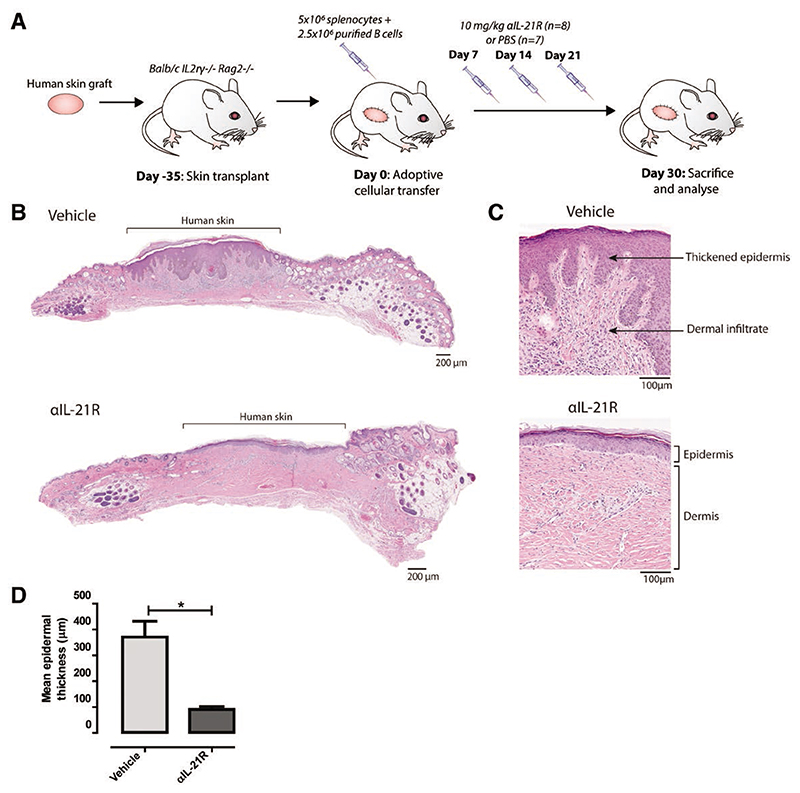
Figure 2. αIL-21R mAb treatment delays rejection of human skin grafts.
A, Treatment strategy used to explore the effect of αIL-21R on rejection of human skin grafts. A circular piece of human skin with a diameter of 1 cm was stitched on the mouse (day 35). After 35 days, the human skin was engrafted and mice then received 5 × 106 splenocytes with 2.5 × 106 enriched quiescent B cells (day 0). αIL-21R was administered at a concentration of 10 mg/kg on days 7, 14, and 21 after adoptive cellular transfer (n = 8 mice). PBS was used as a vehicle control (n = 7 mice); 30 days after adoptive cellular transfer, mice were euthanized for cross-sectional analysis. B, H&E staining of the complete human skin graft of a vehicle-treated animal and an αIL-21R-treated animal. C, ×10 magnification of the H&E staining of the human skin graft of a vehicle and αIL-21R-treated animal. In the vehicle-treated animal (upper panel), thickening of the epidermis and dermal infiltrate was detected in contrast to the αIL-21R-treated animal (lower panel). D, Quantified data of epidermal thickness. Epidermal thickness is presented in μm as a mean of 20 consecutive measurements with the SEM. Vehicle group n = 7; αIL-21R group n = 8 (*P < 0.05). αIL-21R, anti-interleukin-21 receptor antibody; H&E, hematoxylin and eosin.