Abstract
Aims
The aims were to use the rs1229984 variant associated with alcohol consumption as an instrument for alcohol consumption to test the causality of the association of alcohol consumption with hay fever, asthma, allergic sensitization, and serum total IgE.
Design
Observational and Mendelian randomization analyses using genetic variants as unbiased markers of exposure to estimate causal effects, subject to certain assumptions.
Setting
Europe.
Participants
We included a total of 466434 persons aged 15–82 years from 17 population-based studies conducted from 1997–2015.
Measurements
The rs1229984 (ADH1B) was genotyped, alcohol consumption, hay fever and asthma were self-reported. Specific and total IgE were measured from serum samples.
Findings
Observational analyses showed that ever-drinking vs. non-drinking, but not amount of alcohol intake, was positively associated with hay fever and inversely associated with asthma but not with allergic sensitization, or serum total IgE. However, Mendelian randomization analyses did not suggest that the observational associations are causal. The causal odds ratio (OR) per genetically assessed unit of alcohol/week was an OR=0.91 (95% confidence interval (CI): 0.81, 1.02; p=0.101) for hay fever, an OR=0.90 (95% CI: 0.79, 1.02; p=0.095) for asthma, an OR=0.97 (95% CI: 0.80, 1.17; p=0.763) for allergic sensitization, and a 4.7% change (95% CI: -5.5%, 14.9%; p=0.366) for total IgE.
Conclusions
Ever-drinking vs. not drinking was in observational analyses positively associated with hay fever, and negatively associated with asthma. However, the Mendelian randomization results were not consistent with these associations being causal.
Keywords: Alcohol, allergic disease, allergic sensitization, asthma, hay fever
Introduction
Alcohol is a strong immune modulating factor (1, 2). Previous observational studies have shown that moderate and excessive alcohol intake is associated with higher serum levels of total immunoglobulin E (IgE) (3–13). Likewise, alcohol consumption in pregnancy is associated with an increase in cord blood IgE levels from the newborn (14). Some epidemiologic studies have found a positive association between alcohol consumption and allergic sensitization (9, 12) but other studies have not (6, 8, 15). Regarding allergic disease, alcohol consumption was positively associated with the risk of developing perennial allergic rhinitis (16), and alcohol consumption during pregnancy may increase the risk of atopic dermatitis in the offspring (17). In addition, alcohol is a trigger of hypersensitivity reactions and can cause asthma in genetically predisposed individuals (18). The possible mechanisms by which alcohol consumption could affect allergic sensitization and levels of total IgE may include a direct effect of alcohol or its metabolites on lymphocyte subsets with subsequent T helper (Th)1/Th2 cytokine imbalance, or an indirect effect due to alcohol-induced permeability of the gut mucosa to endotoxin of other bacterial products (1, 3, 5, 10, 19).
Causal inference from conventional epidemiologic studies between alcohol consumption and allergic respiratory disease is difficult due to the potential confounding and reverse causation. Mendelian randomization examines causality by using genetic variants, typically single nucleotide polymorphism (SNP), as instruments for exposures. It assumes random allocation of genes from parents to offspring and no direct association between genotype and outcome and will not be associated with the confounding factors that are inherent in conventional observational studies. The enzyme alcohol dehydrogenase (ADH) oxidizes alcohol to acetaldehyde. The more active forms of this enzyme are protective against drinking, because they cause higher levels of acetaldehyde. In European samples, the rs1229984 variant in the ADH1B gene is most strongly associated with alcohol phenotypes, and the protective A-allele frequency is approximately 2–5% in Europeans (20). The rs1229984 has previously been used as instrument for alcohol consumption in Mendelian randomization studies to examine the effect of alcohol consumption in cardiovascular disease (20).
Persons sensitized to inhalant allergens as, measured by serum specific immunoglobulin E (IgE), are at risk of developing allergic respiratory disease. Serum specific IgE positivity to inhalant allergens are an accepted objective tests of allergic respiratory disease in clinical assessment and in epidemiological studies. The aims were to use the rs1229984 variant as an instrument for alcohol consumption to test the causality of the association of alcohol consumption with 1) hay fever, 2) asthma, 3) allergic sensitization, and 4) serum total IgE, in a Mendelian randomization meta-analysis of 466434 participants across 17 studies. We examined the effects for ever-drinkers and non-drinkers separately.
Methods
Design
We performed multicenter traditional observational and Mendelian randomization analyses to determine whether alcohol consumption causally affects allergic respiratory disease, and asthma, and estimate the magnitude of the associations.
Study populations
We included 466434 participants (including 98786 cases of hay fever, 53796 cases of asthma, and 6053 cases of allergic sensitization) of self-reported or genetically determined European ancestry aged ≥16 years from the following 17 studies: The British 1958 birth cohort (1958 BC), Copenhagen City Heart Study (CCHS), the Danish Monitoring of trends and determinants in Cardiovascular Diseases (MONICA) Study (the Dan-Monica10 Study), the Allergy98 Study, Genomics of Overweight Young Adults (GOYA) Males, the Health2006 Study, the Inter99 Study, the Cooperative Health Research in the Region of Augsburg (KORA) Study, the MRC National Survey of Health and Development (NSHD) Study, the 1936 Cohort, the UK Biobank, the Netherlands Epidemiology of Obesity (NEO) Study, the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER), the Rotterdam Study, the Studies of Health in Pomerania (SHIP and SHIP TREND), and the DanFunD Study. Further details of these studies are provided in online Supplementary Material and Supplementary Tables S1-S4.
Measures
Genotype
Rs1229984 is located in the alcohol dehydrogenase 1B gene (ADH1B). It encodes the ADH1B enzyme that is the main metabolizer of alcohol (20). Due to the low prevalence of the rs1229984 A-allele, we recoded the genetic variant according to a dominant model into major homozygotes (alcohol-increasing genotype) vs. heterozygotes and minor homozygotes combined (alcohol-decreasing genotypes). Description of the method for genotyping within each study is provided in supplementary material. Genotype frequencies and Hardy-Weinberg equilibrium levels are shown in Table S3. The minor allele frequency ranged from 0.008–0.070 across studies. The SNP was directly genotyped in each study, except for the DanFunD study, KORA, the 1936 Cohort, the Rotterdam study, and the NEO study (Supplementary Material).
Hay fever, asthma, allergic sensitization, lung function, and serum total IgE
Data on hay fever and asthma were obtained from interviews or questionnaires according to Supplemental Table S4. Our first choice of definition/diagnosis was lifetime/ever, but if not available we used a diagnosis in the past 12 months or longer (Table S4). Allergic sensitization was defined by serum specific IgE positivity to at least one of the tested inhalant allergens (Table S4). We used the cut-offs for positivity generally recommended by the manufacturer. Serum total IgE was measured by a number of assays as detailed in Supplementary Material and Table S2 and S4.
Alcohol intake
We used self-reported alcohol status (non-drinker or ever-drinker) and units of alcohol consumed per week (either by interview or questionnaire), reported at the same time or as close as possible to the assesment of allergic respiratory disease (see Supplementary Material).
Statistical analyses
Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), STATA, version 12 (StataCorp, College Station, TX, USA), the statistical software R, version 3.3.3 (http://www.r-project.org/), and Quanto, version 1.2 (University of Southern California, US). The p-values are two-tailed, and statistical significance was defined as P<0.05. Serum total IgE was log-transformed in the regression analyses to fulfill requirements on normality.
With an assumed protective allele frequency of 2% for the rs1229984 genotype, we calculated the required number of cases and controls among ever-drinkers in Quanto 1.2 (University of Southern California, US) for a range of effect sizes prior to the study. For example, with a power of 0.80 we would need approximately 6000 and 18000 ever-drinkers with and without hay fever, respectively, to detect an odds ratio of 0.85 and 14000 and 42000 ever-drinkers with and without hay fever, respectively, to detect an odds ratio of 0.90 among carriers of the protective allele compared to non-carriers. Likewise, for the natural logarithm of serum total IgE (mean=3.4 and standard deviation=1.5) as outcome, we would need approximately 40000 ever-drinkers to detect an R2=0.0002/ß=-0.10 for carriers vs. non-carriers of the protective allele. The sample size needed to estimate an effect on, e.g., hay fever per genetically determined drink of alcohol with a power of 0.8, type 1 error rate og 0.05, variance in amount of alcohol explained by the SNP of 0.0045, and an OR=1.3 of hay fever per standard deviation of amount of alcohol, was calculated to be N=293025 (21).
The study was performed as a multicenter meta-analysis. We provided the study representatives with an invitation to contribute to the study, an analysis protocol, and a Stata-script to be used in the analyses. The study results were combined in random effects meta-analyses. Participants with missing value in one or more variables were excluded. Analyses were performed in all participants and in strata defined by alcohol status. The main analyses were performed in ever-drinkers. The analyses were adjusted for age, gender, and genetic principal components if available (British 1958 Birth Cohort, the NEO study, and the UK Biobank).
First, observational analyses of the associations of alcohol status and intake with hay fever, asthma, allergic sensitization, and serum total IgE were assessed by logistic and linear regression analyses. Second, Mendelian randomization analyses were performed. The associations of the alcohol-associated SNP and alcohol status, alcohol intake, hay fever, asthma, allergic sensitization, and serum total IgE were assessed using linear and logistic regression. The estimates from each study were meta-analyzed using the ‘metan’ command in Stata. Heterogeneity was examined by the I2 statistic. The instrumental variable (IV) analyses were performed by the “MendelianRandomization” package in R using the inverse variance-weighted method (“mr_ivw”), similar to the ratio method for a single IV. These were performed regardless of statistical significance to show the size of the estimates.
Since the primary definition of hay fever in the UK Biobank also included eczema, we performed additional analyses using different definitions (See Supplementary). A part of the UK Biobank Study, the UK Biobank Lung Exome Variant Evaluation (UK BiLEVE) study, was sampled according to lung function and smoking status, and we performed sensitivity analyses excluding this study (See Supplementary). Analyses including UK Biobank data and 1958 BC where the alcohol genotype was directly genotyped, and adjustments were made for principal components are shown in Supplementary Figures S1–S5.
Results
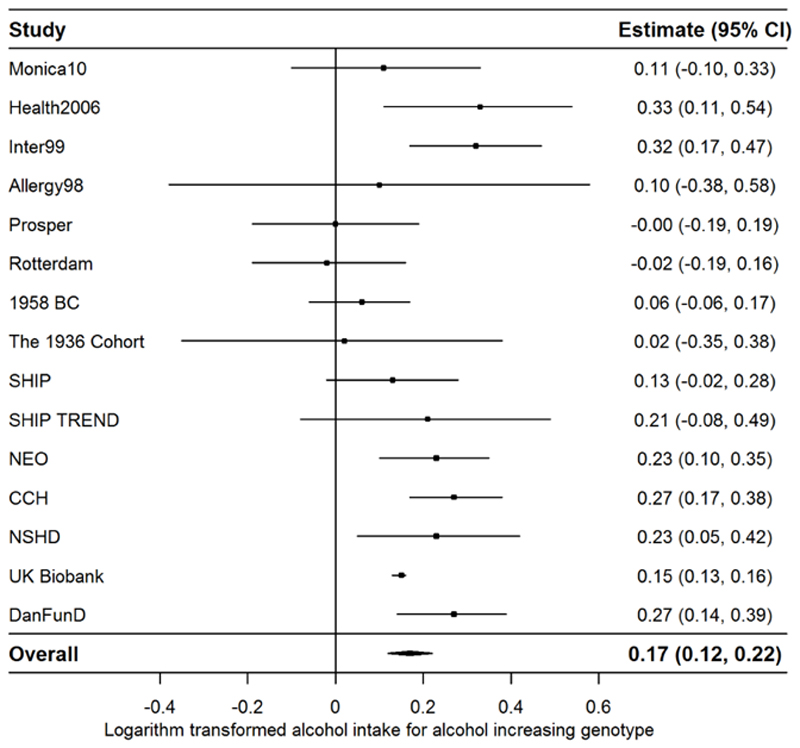
Descriptive statistics for each of the study populations are shown in Table 1 and Supplementary Table S1. Minor allele frequencies and Hardy Weinberg Equilibrium p-values are shown in Supplementary Table S3. There was a statistically significant positive association between the rs1229984 genotype (major homozygotes vs. minor homozygotes and heterozygotes) and higher alcohol intake (logarithmically transformed) (Figure 1).
Table 1. Overview of collaborating studies.
| Study name | Year | N | Age, median (IQR) | % males | Place |
|---|---|---|---|---|---|
| 1936-cohort (33) | 1976–77 | 592 | 60.5 (60.2, 60.8) | 47.3 | Copenhagen, Denmark |
| Monica10 (33) | 1993–94 | 2262 | 52.0 (42.1, 61.9) | 49.7 | Glostrup, Copenhagen, Denmark |
| Allergy98 (33) | 1997–98 | 1148 | 38.0 (28.7, 51.3) | 45.6 | Western Copenhagen, Denmark |
| Inter99 (33) | 1999–01 | 4880 | 45.1 (40.0, 50.2) | 49.0 | Copenhagen area, Denmark |
| Health2006 (34) | 2006–08 | 2818 | 50.0 (40.0, 60.0) | 45.0 | Copenhagen area, Denmark |
| DanFunD (35) | 2012–15 | 7093 | 54 (44, 63) | 46.3 | Copenhagen area, Denmark |
| GOYA Males (36) | 1992–94 | 790 | 46 (41, 53) | 100 | Copenhagen area, Denmark |
| 1958 BC (37) | 2000 | 2420 | 42 (42, 42) | 52.1 | England, Scotland and Wales |
| KORA (38) | 1997–98 | 1255 | 49.0 (39.0, 59.0) | 49.1 | Augsburg, Germany |
| NEO (39) | 2008–12 | 5557 | 57.0 (51.0, 61.0) | 48.3 | Leiden, Netherlands |
| NSHD (40) | 1999 | 2675 | 53 (53, 53) | 49.9 | England, Scotland and Wales |
| Prosper (41) | 1997–99 | 5504 | 75.0 (72.4, 77.9) | 48.3 | Scotland, Ireland, Netherlands |
| UK Biobank (42) | 2006–10 | 407767 | 58 (51, 63) | 46.0 | United Kingdom |
| Rotterdam (43) | 1990–93 | 7977 | 62.5 (58.2, 70.5) | 43.0 | Rotterdam, Netherlands |
| SHIP (44) | 1997–01 | 3725 | 50.0 (36.0, 62.0) | 48.7 | West Pomerania, Germany |
| SHIP TREND (45) | 2008–12 | 986 | 50.0 (40.0, 61.0) | 43.8 | West Pomerania, Germany |
| CCH (46) | 1991–94 | 8985 | 60.5 (47.8, 70.3) | 44.3 | Copenhagen area, Denmark |
Abbreviations: Allergy98, Copenhagen Allergy study; 1958 BC, British 1958 Birth Cohort; CCH, Copenhagen City Heart Study; GOYA, Genomics of extremely Overweight Young Adults; Inter99, Intervention 1999; IQR, interquartile range; KORA, Cooperative Health Research in the Region of Augsburg; NEO, Netherlands Epidemiology of Obesity; NSHD, National Survey of Health and Development; SHIP, Study of Health in Pomerania.
Figure 1.
Random effects meta-analysis of the associations between the rs1229984 genotype (major homozygotes vs. minor homozygotes and heterozygotes) and the logarithm transformed alcohol intake (see heterogeneity in Supplementary Figure 1). Abbreviations: Allergy98, Copenhagen Allergy study; 1958 BC, British 1958 Birth Cohort; CCH, Copenhagen City Heart Study; Inter99, Intervention 1999; NEO, Netherlands Epidemiology of Obesity; NSHD, National Survey of Health and Development; SHIP, Study of Health in Pomerania.
Observational analysis
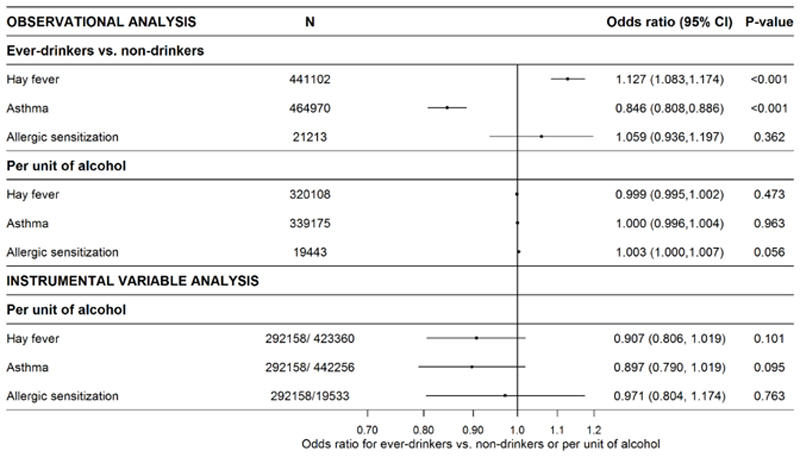
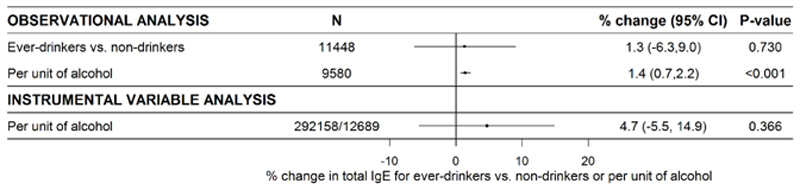
Ever-drinking, but not alcohol intake, was positively associated with hay fever. Ever-drinking, but not alcohol intake, was inversely associated with asthma (Figure 2). The observed associations of ever-drinking with hay fever and asthma, respectively, were rather consistent across studies (Supplementary Figures S6–S7). For alcohol intake and hay fever and asthma, there was some heterogeneity across studies (Supplementary Figures S10–S11). Ever-drinking and alcohol intake were positively, though ever-drinking statistically non-significantly, associated with allergic sensitization (Figure 2), with low heterogeneity across studies (Supplementary Figures S8 and S12). The number of participants in the analyses of allergic sensitization was substantially lower (N=19443–21213, Figure 2) than for hay fever and asthma. Intake of alcohol, but not ever-drinking, was significantly associated with increasing serum total IgE and log(total IgE) (Figure 3).
Figure 2.
Random effects meta-analyses of observational and instrumental variable estimates of the sex- and age-adjusted associations of alcohol status and -intake and genetically assessed alcohol intake, respectively, and hay fever, asthma, and allergic sensitization (see heterogeneity in Supplementary Figure 2–4 and 6–8). Numbers in the instrumental variable analyses refer to SNP-exposure and SNP-outcome associations, respectively. Abbreviations: CI, confidence interval.
Figure 3.
Random effects meta-analyses of observational and instrumental variable estimates of the sex- and age-adjusted and sex-stratified associations of alcohol status and -intake and genetically assessed alcohol intake, respectively, and serum total IgE (see heterogeneity in Supplementary Figure 5 and 9). Numbers in the instrumental variable analyses refer to SNP-exposure and SNP-outcome associations, respectively. Abbreviations: CI, confidence interval; IgE, immunoglobulin E.
Mendelian randomization analysis
The rs1229984 genotype associated with higher alcohol intake (major homozygotes vs. minor homozygotes and heterozygotes) was not significantly associated with hay fever, asthma, allergic sensitization, or log(total IgE) in ever- or non-drinkers (Supplementary Figure S2–S5).
The estimate for the first stage regression (SNP-exposure) among ever-drinkers (calculated in the UK Biobank data) was β=1.324 (standard error (SE)=0.086, N=292158) units of alcohol for alcohol-increasing genotype vs. alcohol-decreasing genotypes. The estimates for the second stage regressions (SNP-outcome) were as follows: hay fever β= -0.130 (SE=0.079); asthma β= -0.144 (SE=0.086); allergic sensitization β= -0.038 (SE=0.128); and log(total IgE) β=0.062 (SE=0.069).
There was no clear evidence that genetically assessed intake of alcohol was associated with risk of hay fever, asthma, or allergic sensitization. The causal estimates, per unit of alcohol consumed, were an OR=0.907 (95% CI: 0.806,1.019; p=0.101) for hay fever, an OR=0.897 (95% CI: 0.790, 1.019; p=0.095) for asthma, and an OR=0.971 (95% CI: 0.804, 1.174; p=0.763) for allergic sensitization.
Genetically assessed higher alcohol intake was not associated with total IgE (Figure 3). The causal estimate of log(total IgE) for ever-drinkers was β=0.047 (95% CI: -0.055, 0.149; p=0.366) per unit of alcohol, which reflects an approximate 4.7% change (95% CI: -5.5%, 14.9%; p=0.366) in total IgE per unit of alcohol consumed (Figure 3).
Analyses including UK Biobank data and 1958 BC where the alcohol genotype was directly genotyped, and adjustments were made for principal components (Supplementary Figures S1–S5) let to similar conclusions.
The primary definition of hay fever in UK Biobank suggested an OR=0.987 (95% CI: 0.952, 1.023, p=0.474, N=394883) for ever-drinkers with alcohol increasing genotype. Using self-reported hay fever medication as alternative definition of hay fever (see Supplementary Material) suggested an OR=0.977 (95% CI: 0.876, 1.089, p=0.671, N=394883). Using self-reported hay fever classified as serious illness as alternative definitions of hay fever suggested an OR=1.011 (95% CI: 0.934, 1.093, p=0.793, N=394883). Hay fever in UK Biobank without the UK Biobank Lung Exome Variant Evaluation (UK BiLEVE) study (22) (see Supplementary Material) suggested an OR= 0.984 (95% CI: 0.947, 1.022, p=0.398, N=351833).
Asthma in the UK Biobank without BiLEVE suggested an OR=0.989 (95% CI: 0.939, 1.041, p=0.666, N=351833), whereas asthma in the entire UK Biobank suggested an OR= 0.995 (95% CI: 0.949, 1.044, p=0.845, N=394883) for the alcohol increasing genotype.
Discussion
We found that ever-drinking was observationally positively associated with hay fever and inversely associated with asthma, but there was no clear evidence of association with allergic sensitization or total IgE, and in Mendelian randomisation analyses, genetically assessed intake of alcohol was not associated with hay fever, asthma, allergic sensitization, or serum total IgE, suggesting that the observational associations are not causal.
Oldenburg et al found that alcohol feeding decreased airway hyper-responsiveness and allergic airway inflammation in allergic mice, suggesting that there may be an important role for alcohol in the modulation of asthma (23). Few studies have examined the possible bronchodilator effect of alcohol in humans (24, 25). Ayres et al found that intake of 40 ml of sherry was consistent with an increase in peak expiratory flow rate in 19 patients with asthma but not in 16 controls (25). This bronchodilation was more marked in patients with peak flow rate less than 50% of the predicted rate. Lieberoth et al found that alcohol intake was associated with incident asthma in adults with a U-shaped association where the lowest risk of asthma was observed in the group with a moderate intake of alcohol (26). There is evidence that acetaldehyde increases histamine release from mast cells in both animal and human studies (10). In Japanese populations, in which a genetic variant causing high levels of acetaldehyde is very common, alcohol is in these genetically predisposed individuals accompanied by bronchoconstriction and flushing (alcohol-induced asthma and Oriental flushing) following alcohol intake. Whether the alcohol-induced Th2-skewing of the human immune response is mediated trough acetaldehyde is not fully known.
A study by Siu et al found that light to moderate alcohol drinkers had better lung function than abstainers did. This was independent of smoking and evident lung or heart disease suggesting that drinking moderate amounts of alcohol may benefit lung function (27). Frantz et al found that alcohol and heavy drinking in particular had a negative effect on lung function in smokers (28). However, Sparrow et al found that alcohol consumption was not associated with baseline or follow-up levels of FEV1 or FVC in 1,067 men (29).
Several cross-sectional studies have reported a positive association between alcohol intake and allergic sensitization (9, 11, 12). However, Assing et al found no association between alcohol intake and skin prick test positivity among 1668 students (8). The association between alcohol intake and incident allergic sensitization in cohort studies is even more controversial (6, 15). In a study of 5870 women aged 20–29 years, Bendtsen et al (16) found that self- reported alcohol consumption was positively associated with the risk of developing perennial allergic rhinitis but not seasonal allergic rhinitis. The association of moderate and excessive alcohol intake with higher serum levels of total IgE is more consistent (4–6, 9, 12, 13). A high consumption of alcohol may also increase IgE sensitization to cross-reactive carbohydrate determinants, and specific IgE results should be interpreted with caution in heavy alcohol drinkers (30). In addition, experimental alcohol administration to rodents induces an increase of serum IgE concentrations (19). Moreover, cessation of alcohol consumption in heavy drinkers is shortly followed by a decrease of serum IgE concentrations (4, 13). In a Mendelian Randomization study of 111,408 persons from the general population, Nordestgaard et al found that genetically assessed higher alcohol intake was positively associated with total IgE (but not with allergic disease) (31).
In accordance with previous studies, we found that the rs1229984 was strongly associated with drinking status and alcohol intake which shows that rs1229984 is well-suited as a genetic instrument of alcohol intake (20). We used a standardized analytical protocol to increase reliability and robustness of the findings. To avoid confounding by population stratification, we included Europeans only and adjusted for population structure using principal components when possible. Since this covered most of the participants, we regard confounding by stratification to be of minor concern. We used objective markers of allergic sensitization, serum total IgE and lung function which may be more reliable than self-reported measures in certain situations. Using Mendelian randomization has several shortcomings, e.g., the observational analysis of ever versus never drinkers cannot be mimicked using this approach. Since the genotype explains only a small part of the variation in alcohol consumption, Mendelian randomization has lower power that traditional observational studies for comparable study sizes, i.e. Mendelian randomization studies need very large samples to be adequately powered. A limitation of the study is the use of mostly self-reported hay fever and asthma rather than clinical diagnoses or measurements. A non-differential misclassification of a binary outcome is likely to attenuate associations towards the null. We also defined alcohol consumption from self-report but the validity compared with alternative measurements has previously been found to be reasonable (32). The hay fever variable in the UK Biobank included eczema. Therefore, we assessed two additional hay fever variables from the UK Biobank with similar results which indicates that the misclassification did not substantially bias our results. The main weaknesses of the additional hay fever variables were the fact that one was measured at follow-up in 2015 and in a subgroup only, limiting the available sample size; and the other was based on self-reported medication rather than a doctor-diagnosed hay fever. Of note, alcohol intake did not distinguish between, e.g., beer and wine. We may have lacked power to show a similar association between alcohol and serum total IgE. It would be of interest to also study extreme exposure to alcohol, as seen in alcoholics. However, such individuals would also differ more substantially from the rest of the population with regard to potential confounders such as genetics of alcohol dependence and socioeconomic factors. The present study focused on the exposure levels in the normal range and in a population-based setting. It is important to acknowledge that the present study does not investigate effects of exposure to alcohol in childhood, before use of alcohol, and thus only relates to cases of asthma/hay fever that develop after start of alcohol use. However, it is increasingly being recognized that many cases of hay fever and asthma have their onset in adolescence or later in adulthood. Moreover, a factor that exacerbate or influence duration of a disease could also increase the prevalence of disease.
In conclusion, observational analyses found that ever-drinking vs. non-drinking was positively associated with hay fever and inversely with asthma but not with allergic sensitization, and serum total IgE. In contrast, genetic predisposition to consume more alcohol did not affect risk of hay fever, asthma, allergic sensitization, or serum levels of total IgE. The discrepancy of results between observational and Mendelian randomization analyses could be due to potential residual confounding, e.g., by socioeconomic factors or lifestyle factors. Our results challenge the concept of a risk-increasing effect of alcohol consumption on allergic disease and asthma.
Ethics
All the included studies were approved by local ethics committees, and all participants gave their informed consent.
Supplementary Material
Funding and acknowledgements
Tea Skaaby was supported by grants from the Lundbeck Foundation (Grant number R165-2013-15410 and R219-2016-471), the Harboe Foundation (Grant number 16152), the A.P. Møller Foundation for the Advancement of Medical Science (Grant number 15-363), Aase and Einar Danielson’s Foundation (Grant number 10-001490) and the Weimann’s grant. This research has been conducted using the UK Biobank Resource (Application number: 17765). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk).
Footnotes
Conflicts of interest
Lies Lahousse reports expert consultation for Boehringer Ingelheim GmbH and Novartis, grants from AstraZeneca and Chiesi, grants and non-financial support from European Respiratory Society and Belgian Respiratory Society, all outside the submitted work. The remaining authors declare that they have no conflicts of interest.
Author contributions
All authors were involved in the conception and design of the study or the collection and processing of samples. TS conducted data analyses and wrote the initial manuscript. All authors were involved in critical appraisal and revision of the manuscript, and all authors approved the final version.
References
- 1.Pasala S, Barr T, Messaoudi I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015;37(2):185–97. [PMC free article] [PubMed] [Google Scholar]
- 2.Szabo G, Saha B. Alcohol's Effect on Host Defense. Alcohol Res. 2015;37(2):159–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez-Santalla MJ, Vidal C, Vinuela J, Perez LF, Gonzalez-Quintela A. Increased serum IgE in alcoholics: relationship with Th1/Th2 cytokine production by stimulated blood mononuclear cells. Alcohol ClinExpRes. 2001;25(8):1198–205. doi: 10.1111/j.1530-0277.2001.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Quintela A, Vidal C, Gude F, Tome S, Lojo S, Lorenzo MJ, et al. Increased serum IgE in alcohol abusers. ClinExpAllergy. 1995;25(8):756–64. doi: 10.1111/j.1365-2222.1995.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Quintela A, Vidal C, Lojo S, Perez LF, Otero-Anton E, Gude F, et al. Serum cytokines and increased total serum IgE in alcoholics. AnnAllergy Asthma Immunol. 1999;83(1):61–7. doi: 10.1016/S1081-1206(10)63514-4. [DOI] [PubMed] [Google Scholar]
- 6.Linneberg A, Petersen J, Nielsen NH, Madsen F, Frolund L, Dirksen A, et al. The relationship of alcohol consumption to total immunoglobulin E and the development of immunoglobulin E sensitization: the Copenhagen Allergy Study. ClinExpAllergy. 2003;33(2):192–8. doi: 10.1046/j.1365-2222.2003.01515.x. [DOI] [PubMed] [Google Scholar]
- 7.Vally H, Thompson PJ. Alcoholic drink consumption: a role in the development of allergic disease? ClinExpAllergy. 2003;33(2):156–8. doi: 10.1046/j.1365-2222.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- 8.Assing K, Bodtger U, Linneberg A, Malling HJ, Poulsen LK. Association between alcohol consumption and skin prick test reactivity to aeroallergens. AnnAllergy Asthma Immunol. 2007;98(1):70–4. doi: 10.1016/S1081-1206(10)60862-9. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Quintela A, Gude F, Boquete O, Rey J, Meijide LM, Suarez F, et al. Association of alcohol consumption with total serum immunoglobulin E levels and allergic sensitization in an adult population-based survey. ClinExpAllergy. 2003;33(2):199–205. doi: 10.1046/j.1365-2222.2003.01582.x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Quintela A, Vidal C, Gude F. Alcohol, IgE and allergy. AddictBiol. 2004;9(3–4):195–204. doi: 10.1080/13556210412331292235. [DOI] [PubMed] [Google Scholar]
- 11.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Factors related to allergic sensitization to aeroallergens in a cross-sectional study in adults: The Copenhagen Allergy Study. ClinExpAllergy. 2001;31(9):1409–17. doi: 10.1046/j.1365-2222.2001.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.Vidal C, Armisen M, Dominguez-Santalla MJ, Gude F, Lojo S, Gonzalez-Quintela A. Influence of alcohol consumption on serum immunoglobulin E levels in atopic and nonatopic adults. Alcohol ClinExpRes. 2002;26(1):59–64. [PubMed] [Google Scholar]
- 13.Hallgren R, Lundin L. Increased total serum IgE in alcoholics. Acta MedScand. 1983;213(2):99–103. doi: 10.1111/j.0954-6820.1983.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 14.Bjerke T, Hedegaard M, Henriksen TB, Nielsen BW, Schiotz PO. Several genetic and environmental factors influence cord blood IgE concentration. PediatrAllergy Immunol. 1994;5(2):88–94. doi: 10.1111/j.1399-3038.1994.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 15.Linneberg A, Friedrich N, Husemoen LL, Thuesen B, Gonzalez-Quintela A, Vidal C, et al. Incidence and remission of specific IgE aeroallergen sensitization from age of 40 to 60 years, and association with alcohol consumption. IntArchAllergy Immunol. 2010;151(2):142–8. doi: 10.1159/000236004. [DOI] [PubMed] [Google Scholar]
- 16.Bendtsen P, Gronbaek M, Kjaer SK, Munk C, Linneberg A, Tolstrup JS. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. ClinExpAllergy. 2008;38(7):1179–85. doi: 10.1111/j.1365-2222.2008.02945.x. [DOI] [PubMed] [Google Scholar]
- 17.Linneberg A, Petersen J, Gronbaek M, Benn CS. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy. 2004;34(11):1678–83. doi: 10.1111/j.1365-2222.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 18.Linneberg A, Gonzalez-Quintela A, Vidal C, Jorgensen T, Fenger M, Hansen T, et al. Genetic determinants of both ethanol and acetaldehyde metabolism influence alcohol hypersensitivity and drinking behaviour among Scandinavians. Clin ExpAllergy. 2010;40(1):123–30. doi: 10.1111/j.1365-2222.2009.03398.x. [DOI] [PubMed] [Google Scholar]
- 19.Starkenburg S, Munroe ME, Waltenbaugh C. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcohol ClinExpRes. 2001;25(8):1221–30. [PubMed] [Google Scholar]
- 20.Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brion M-JA, Shakhbazov K, Visscher PM. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Artigas MS, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet RespirMed. 2015;3(10):769–81. doi: 10.1016/S2213-2600(15)00283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldenburg PJ, Poole JA, Sisson JH. Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice. AmJPhysiol Lung Cell MolPhysiol. 2012;302(3):L308–L15. doi: 10.1152/ajplung.00077.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayres J, Clark TJ. Alcohol in asthma and the bronchoconstrictor effect of chlorpropamide. BrJDisChest. 1982;76(1):79–87. [PubMed] [Google Scholar]
- 25.Ayres J, Ancic P, Clark TJ. Airways responses to oral ethanol in normal subjects and in patients with asthma. JRSocMed. 1982;75(9):699–704. doi: 10.1177/014107688207500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberoth S, Backer V, Kyvik KO, Skadhauge LR, Tolstrup JS, Gronbaek M, et al. Intake of alcohol and risk of adult-onset asthma. Respir Med. 2012;106(2):184–8. doi: 10.1016/j.rmed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Siu ST, Udaltsova N, Iribarren C, Klatsky AL. Alcohol and lung airways function. PermJ. 2010;14(1):11–8. doi: 10.7812/tpp/09-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frantz S, Wollmer P, Dencker M, Engstrom G, Nihlen U. Associations between lung function and alcohol consumption--assessed by both a questionnaire and a blood marker. Respir Med. 2014;108(1):114–21. doi: 10.1016/j.rmed.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 29.Sparrow D, Rosner B, Cohen M, Weiss ST. Alcohol consumption and pulmonary function. A cross-sectional and longitudinal study. Am Rev Respir Dis. 1983;127(6):735–8. doi: 10.1164/arrd.1983.127.6.735. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Quintela A, Garrido M, Gude F, Campos J, Linneberg A, Lojo S, et al. Sensitization to cross-reactive carbohydrate determinants in relation to alcohol consumption. ClinExpAllergy. 2008;38(1):152–60. doi: 10.1111/j.1365-2222.2007.02863.x. [DOI] [PubMed] [Google Scholar]
- 31.Lomholt FK, Nielsen SF, Nordestgaard BG. High alcohol consumption causes high IgE levels but not high risk of allergic disease. JAllergy ClinImmunol. 2016;138(5):1404–13. doi: 10.1016/j.jaci.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 33.Osler M, Linneberg A, Glumer C, Jorgensen T. The cohorts at the Research Centre for Prevention and Health, formerly 'The Glostrup Population Studies'. IntJEpidemiol. 2011;40(3):602–10. doi: 10.1093/ije/dyq041. [DOI] [PubMed] [Google Scholar]
- 34.Thuesen BH, Cerqueira C, Aadahl M, Ebstrup JF, Toft U, Thyssen JP, et al. Cohort Profile: the Health2006 cohort, research centre for prevention and health. IntJ Epidemiol. 2014;43(2):568–75. doi: 10.1093/ije/dyt009. [DOI] [PubMed] [Google Scholar]
- 35.Dantoft TM, Ebstrup JF, Linneberg A, Skovbjerg S, Madsen AL, Mehlsen J, et al. Cohort description: The Danish study of Functional Disorders. ClinEpidemiol. 2017;9:127–39. doi: 10.2147/CLEP.S129335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paternoster L, Evans DM, Nohr EA, Holst C, Gaborieau V, Brennan P, et al. Genome-wide population-based association study of extremely overweight young adults--the GOYA study. PLoSOne. 2011;6(9):e24303. doi: 10.1371/journal.pone.0024303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power C, Elliott J. Cohort profile: 1958 British birth cohort (National Child Development Study) IntJEpidemiol. 2006;35(1):34–41. doi: 10.1093/ije/dyi183. [DOI] [PubMed] [Google Scholar]
- 38.Schafer T, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Wichmann HE, et al. School education and allergic sensitization in adults. Allergy. 2001;56(12):1206–10. doi: 10.1034/j.1398-9995.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- 39.de MR, den HM, Rabelink TJ, Smit JW, Romijn JA, Jukema JW, et al. The Netherlands Epidemiology of Obesity (NEO) study: study design and data collection. EurJEpidemiol. 2013;28(6):513–23. doi: 10.1007/s10654-013-9801-3. [DOI] [PubMed] [Google Scholar]
- 40.Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, et al. Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. IntJEpidemiol. 2011;40(1):e1–e9. doi: 10.1093/ije/dyq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. AmJCardiol. 1999;84(10):1192–7. doi: 10.1016/s0002-9149(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 42.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoSMed. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofman A, Brusselle GG, Darwish MS, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2016 objectives and design update. EurJEpidemiol. 2015;30(8):661–708. doi: 10.1007/s10654-015-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John U, Greiner B, Hensel E, Ludemann J, Piek M, Sauer S, et al. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. SozPraventivmed. 2001;46(3):186–94. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- 45.Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. Cohort profile: the study of health in Pomerania. IntJEpidemiol. 2011;40(2):294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 46.Schnohr P, Jensen G, Lange P, Scharling H. The Copenhagen City Heart Study - • sterbrounders›gelsen. Tables with data from the third examination 1991-94. European Heart Journal suppl. 2001;3:H1–H83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.