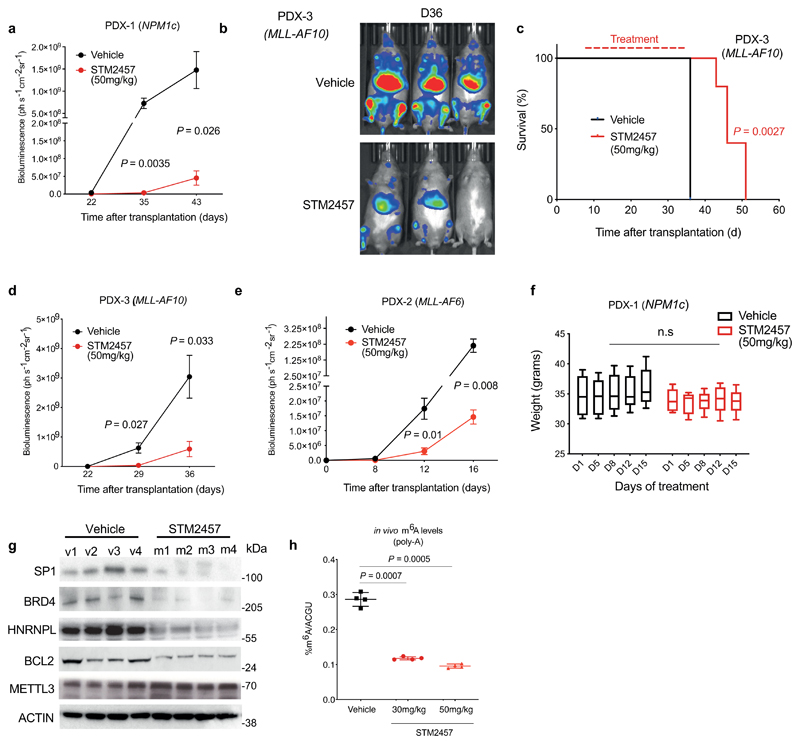
Extended Data Figure. 6. STM2457 shows high efficacy and strong target engagement in PDX models.
a) Quantification of luminescence for the animal experiment depicted in Fig. 4a (mean ± s.d, n=5). b) Bioluminescence imaging of mice transplanted with AML PDX-3 (MLL-AF10) treated with vehicle or 50 mg/kg STM2457 (n=5). c) Kaplan-Meier survival of AML PDX-3 (MLL-AF10) following 12 consecutive treatments with vehicle or 50 mg/kg STM2457 at indicated times (n=5). d) Quantification of luminescence for the animal experiment depicted in Extended Data Fig. 4c (mean ± s.d, n=5). e) Quantification of luminescence for the animal experiment depicted in Fig. 4c (mean ± s.d, n=5). f) Body weight for the animal experiment depicted in Fig. 4a (n=5). Statistical significance was determined by two-tailed Mann–Whitney U test and box plots showing median, IQR and extremes. g) Western blot for SP1, BRD4, HNRNPL, BCL2, METTL3 and ACTIN protein levels in AML PDX-3 (MLL-AF6) treated with vehicle or 50 mg/kg STM2457 (n=4). h) RNA-mass spectrometry quantification of m6A levels on poly-A+-enriched RNA from bone marrow of AML PDX-3 (MLL-AF10) treated in vivo with vehicle, 30 mg/kg STM2457 or 50 mg/kg STM2457 (mean ± s.d., n=4). D, day; n.s. not significant; two-tailed Student’s t-test; Log-rank (Mantel–Cox) test was used for survival comparisons.