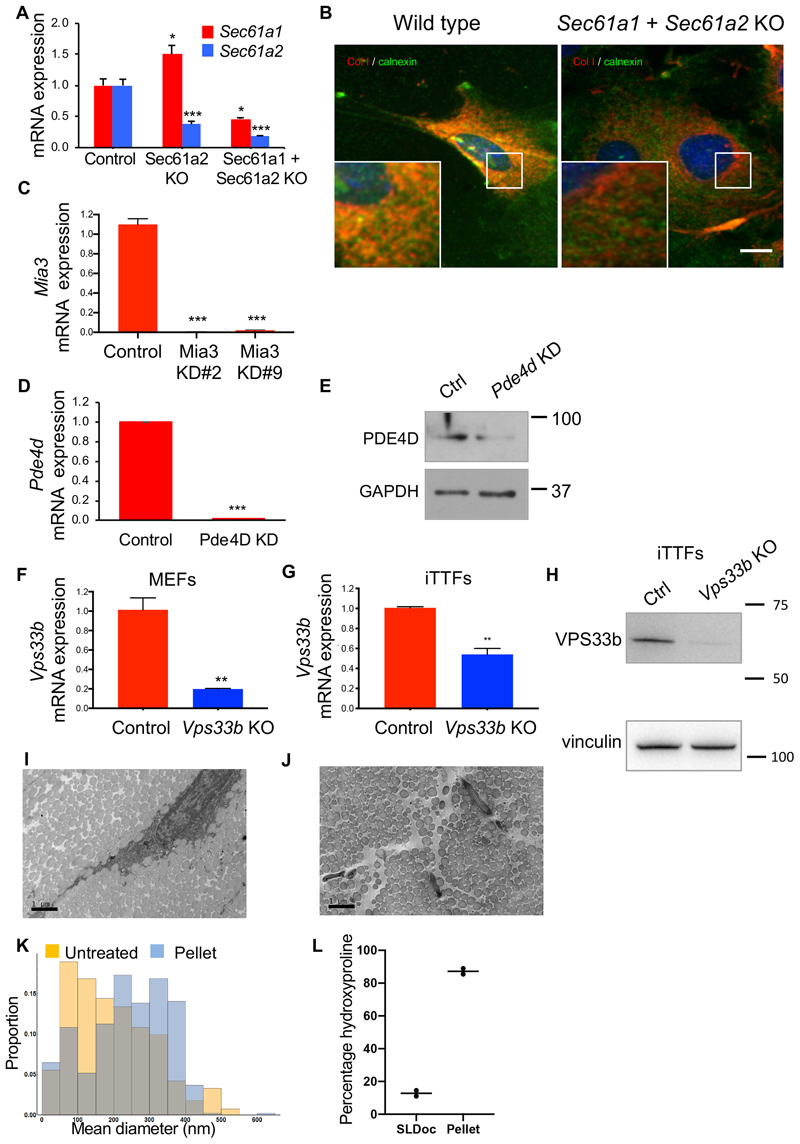
Extended Data Fig. 6. Secretory pathway protein knockdown.
a, Relative expression of Sec61a1 and Sec61a2 mRNA was reduced in MEFs after CRISPR- Cas9-mediated deletion of both Sec61a1 and Sec61a2 compared to control wild-type MEFs (representative results from n = 3 biological repeats; mean and SD errors are shown *p = 0.0159, p = 0.0143 for Sec61a1 levels; ***p = 0.0007, p = 0.0002 for Sec61a2 levels, two-tailed unpaired t-test). b, Immunofluorescence analysis of collagen-I entry into the ER (detected via the marker calnexin) showed little co-localization of collagen-I and calnexin in Sec61a1 and Sec61a2 KO MEFs compared to wild-type MEFs. c, Relative expression of Mia3 mRNA was reduced in MEFs treated with siRNA targeting Mia3 compared to cells treated with scrambled control siRNA (representative results each showing similar degree of knockdown, n = 3 independent experiments, mean and SD errors are shown; ***p = 0.0004 for KD#2 and #9, two-tailed unpaired t-test). d, Relative expression of Pde4d mRNA was reduced in MEFs treated with siRNA targeting Pde4d compared to cells treated with scrambled control siRNA (n = 3 independent experiments; mean and SD errors shown, ***p = 0.0001, two-tailed unpaired t-test). e, Western blot analysis of MEFs treated with siRNAs targeting Pde4d showed depletion of PDE4D protein (Pde4d KD). Levels of GAPDH protein served as a loading control. f, Relative expression of Vps33b mRNA was significantly reduced in MEFs treated with CRISPR/Cas9n targeting Vps33b compared to control. Controls were wild-type MEFs that underwent electroporation with Cas9 protein but without gRNA (n = 3 biologically independent experiments; mean and SD errors are shown, **p = 0.021, two-tailed t-test). g, Relative expression of Vps33b mRNA in iTTFs after CRISPR/Cas9- mediated deletion of Vps33b (n = 3 biologically independent experiments; mean and SD errors shown, **p = 0.024, two-tailed t-test). h, Western blot analysis of VPS33b protein from Vps33b KO immortalized tail tendon fibroblasts (iTTFs). Vinculin protein served as a loading control. i, TEM of mouse tail tendons prepared in PBS, n = 2. Bar, 1 μm. j, TEM of mouse tail tendons after disruption in SL-DOC and collection by centrifugation, n = 2. Bar, 1 μm. k, Diameter distribution of collagen fibrils in unextracted tendon (untreated) and after extraction by SL-DOC (pellet). l, Hydroxyproline content of dissected tail tendons from mice incubated with SL-DOC. Data are expressed in percentage of total collagen extracted from tendons demonstrating that SL-DOC extracts ~ 18% of total collagen in the tendon (n = 2 animals). See also Statistical Source Extended Data Fig. 6 and Unprocessed Blots Extended Fig. 6. Source data