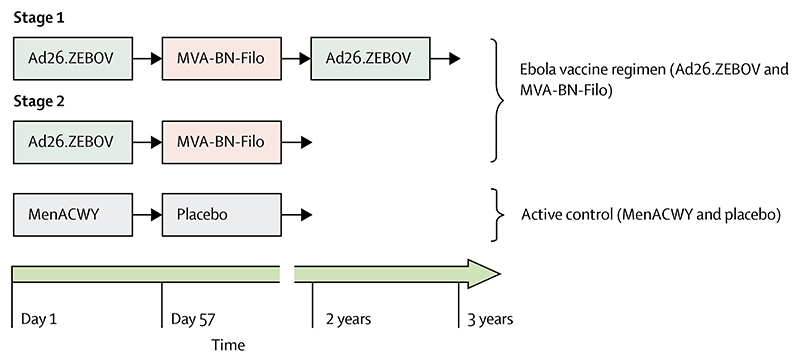
Figure 1. Study design.
Vaccine doses were 5×1010 viral particles for Ad26.ZEBOV, 1×108 infectious units for MVA-BN-Filo, 0·5 mL reconstituted vaccine solution for MenACWY, and 0·5 mL of 0·9% sodium chloride solution for the placebo. Ad26. ZEBOV=adenovirus type 26 vector-based vaccine encoding the Ebola virus glycoprotein. MenACWY=meningococcal quadrivalent (serogroups A, C, W135, and Y) conjugate vaccine. MVA-BN-Filo=modified vaccinia Ankara vector-based vaccine, encoding glycoproteins from the Ebola virus, Sudan virus, and Marburg virus, and the nucleoprotein from the Tai Forest virus.