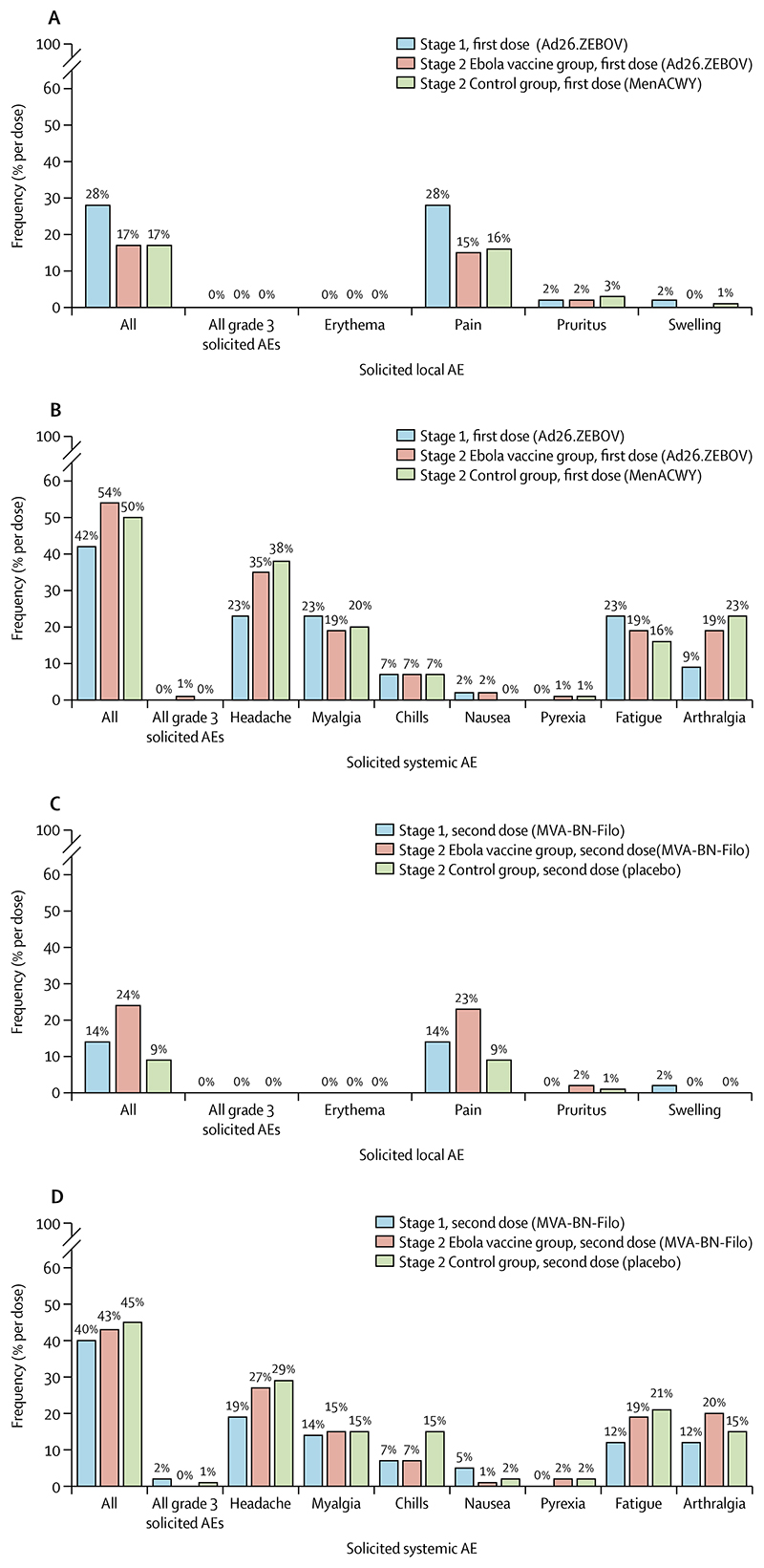
Figure 3. Solicited AEs after vaccination in stage 1 and stage 2 participants.
Solicited local (A) and systemic (B) AEs after the first dose, and solicited local (C) and systemic (D) AEs after the second dose. Solicited AEs were observed during the period of 7 days after vaccination. Grade 3 solicited AEs were severe AEs requiring medical attention, but which were not immediately life-threatening. Ad26.ZEBOV=adenovirus type 26 vector-based vaccine encoding the Ebola virus glycoprotein. MenACWY=meningococcal quadrivalent (serogroups A, C, W135, and Y) conjugate vaccine. MVA-BN-Filo=modified vaccinia Ankara vector-based vaccine, encoding glycoproteins from the Ebola virus, Sudan virus, and Marburg virus, and the nucleoprotein from the Tai Forest virus.