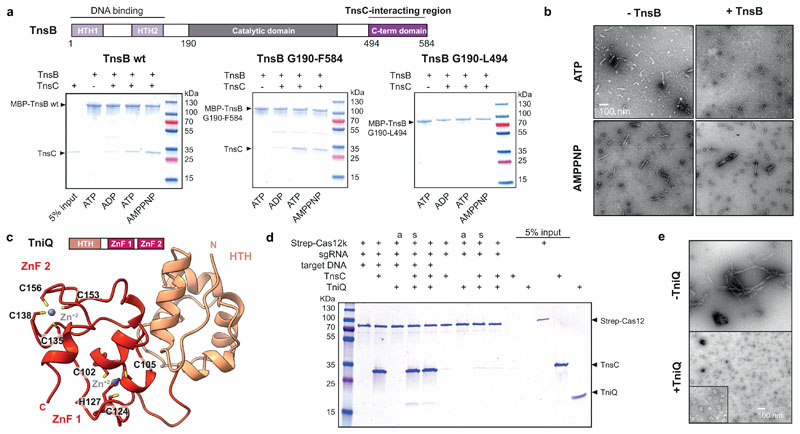
Fig. 4. Functional interactions and roles of ShCAST components.
a, Domain organization of ShTnsB (top panel). HTH, helix turn helix motif. Co-precipitation of ShTnsC by ShTnsB constructs fused to maltose binding protein (MBP) in the presence of different nucleotides (bottom panel). b, Representative negative stain EM micrographs of ShTnsC-dsDNA complexes in the presence of ShTnsB and different nucleotides. Magnification, 98,000 x. c, Top: Domain architecture of ShTniQ. ZnF, zinc-finger motif. Bottom: Crystal structure of ShTniQ. Side chains of zinc-coordinating residues are shown as sticks. d, Co-precipitation of ShTnsC and ShTniQ by immobilized StrepII-fused Cas12k-sgRNA-target DNA complex. a, TnsC and TniQ components were added together; s, sequential addition. e, Representative negative stain EM micrographs of ShTnsC in the presence of dsDNA, ATP and ShTniQ. Magnification of 68,000 x for main micrographs, 120,000 x for insert. Experiments in a, b, d and e were repeated twice independently with similar results. For gel source data, see Supplementary Figure 1.