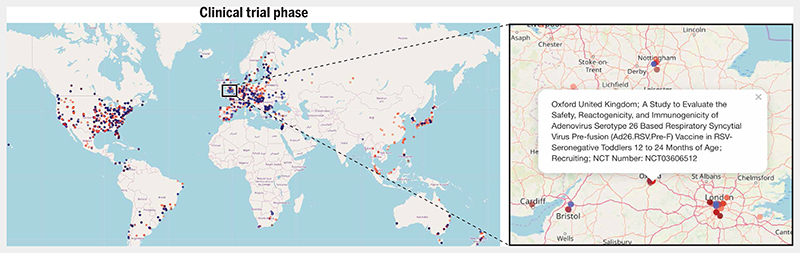
Fig. 3. Global distribution of RSV clinical trials at different phases.
Dots on the global map are color-coded according to the clinical trial phase: Phase 1 trials are shown in maroon, combined phase 1/2 trials are shown in dark red, and phase 2 trials are shown in light red. Combined phase 2/3 trials are shown in light blue and phase 3 trials are shown in dark blue. Purple dots indicate phase 4 trials. Dots are in translucent colors to prevent overlapping dots (depicting proximal trial sites) from obscuring each other. Clicking on the blue box connects to an interactive map (https://rsvclinicaltrials.org/phase.html) where each clickable dot produces a popup box containing detailed information about a particular study site. Here, the popup box provides details of a phase 1/2 trial of an adenovirus vector RSV vaccine in seronegative toddlers aged 12 to 24 months conducted at a trial site in Oxford, UK. Source data for this interactive map were downloaded from clinicaltrials.gov (in December 2019). The search term used in the “condition or disease” field was “Respiratory Syncytial Virus”. All clinical trials that fulfilled this search criterion were added to the source data file that was used to generate the maps.