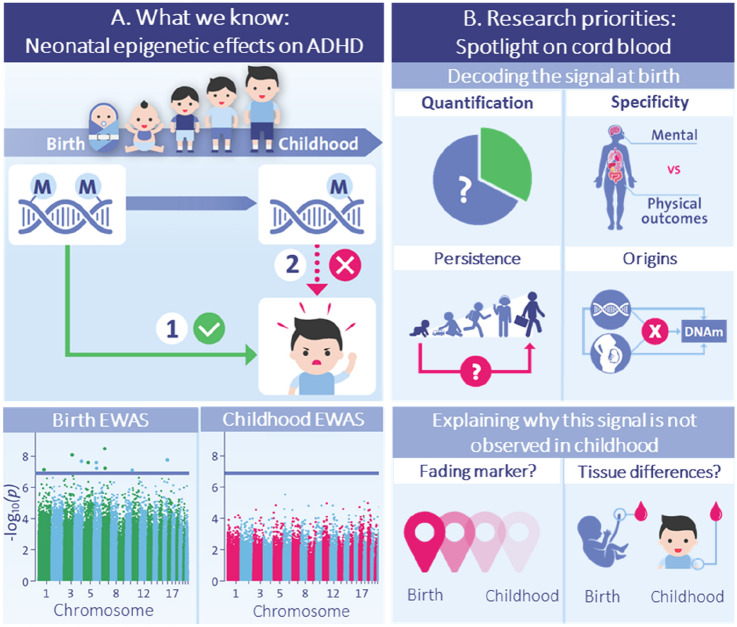
Fig. 2.
Key gap 1: Explaining the neonatal epigenetic signal for ADHD risk. a Top panel: General pattern of epigenetic timing effects observed by EWAS studies in population-based birth cohorts [64, 72], indicating that (1) DNAm patterns at birth prospectively associate with ADHD symptoms in childhood; and (2) DNAm patterns in childhood do not associate cross-sectionally with ADHD symptoms in childhood. Bottom panel: EWAS Manhattan plots from the meta-analysis by Neumann et al. [72] showing that differences in signal are evident both in terms of the identification of prospective, but not cross-sectional, genome-wide significant associations (i.e., ‘dots’ above the blue genome-wide correction line), as well as the overall association ‘signal’ detected across the genome at birth versus in childhood (i.e., height of the bars in the Manhattan plot). b Top panel: Future research will be needed to characterize key properties of the epigenetic signal detected at birth, in order to evaluate its potential as a possible biomarker for early risk detection, pre-symptom manifestation, including (1) how much variance in ADHD it explains (quantification); (2) to what extent this signal is specific to ADHD compared with other child mental and physical outcomes (specificity); (3) whether this signal at birth continues to predict ADHD and related outcomes in adulthood (persistence); and (4) what genetic and environmental influences drive this signal in the first place (origins). Bottom panel: In future, studies will also need to clarify why the epigenetic signal identified at birth is no longer detected from DNAm in childhood, for example due to ‘fading’ predictive power (e.g. DNAm at birth may tag genetic or prenatal influences on ADHD, with this signal becoming noisier in time due to the accumulation of postnatal influences on DNAm) or tissue and cell-type differences between time points (e.g., with cord blood containing unique types of multipotent cells that disappear rapidly after birth, potentially explaining why the signal at birth is not detected in peripheral blood later in life). ADHD attention-deficit/hyperactivity disorder, EWAS epigenome-wide association studies, DNAm DNA methylation