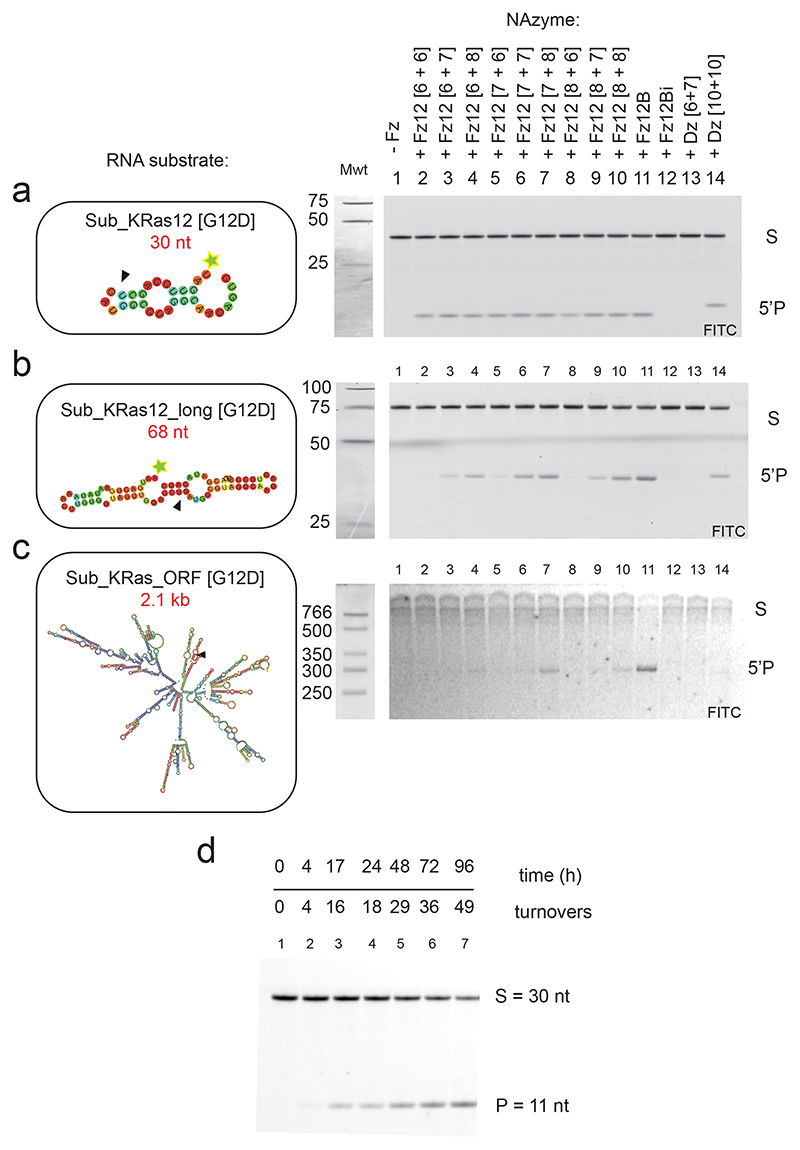
Extended Data Fig. 3. Engineering the KRAS G12D-targeting XNAzyme to invade long RNA substrates.
(a-c) Urea-PAGE gels showing activity of variants of XNAzyme FR6_1_KRas12 (“Fz12”)(1.25 uM) with alternative length substrate-binding arms as indicated, and FR6_1_KRas12B (“Fz12B”)(which has 10 + 10, partially FANA-PS modified binding arms, see Fig. 3), on alternative length RNA substrates (0.25 uM): (a) Sub_KRas12 (30 nt), (b) Sub_KRas12_long (68 nt) and Sub_KRas_ORF (2.1kb), a synthetic transcript comprising the full KRAS ORF and UTRs (1.5 h, 37 °C, pH 7.4, 1 mM Mg2+). (d) Urea-PAGE gel showing Fz12B (10 nM) performing multiple turnover catalysis of Sub_KRas12 [G12D] (1 uM) RNA cleavage (37 °C, pH 7.4, 1 mM Mg2+). Substrate secondary structure diagrams were generated using RNAfold (Methods reference 3).