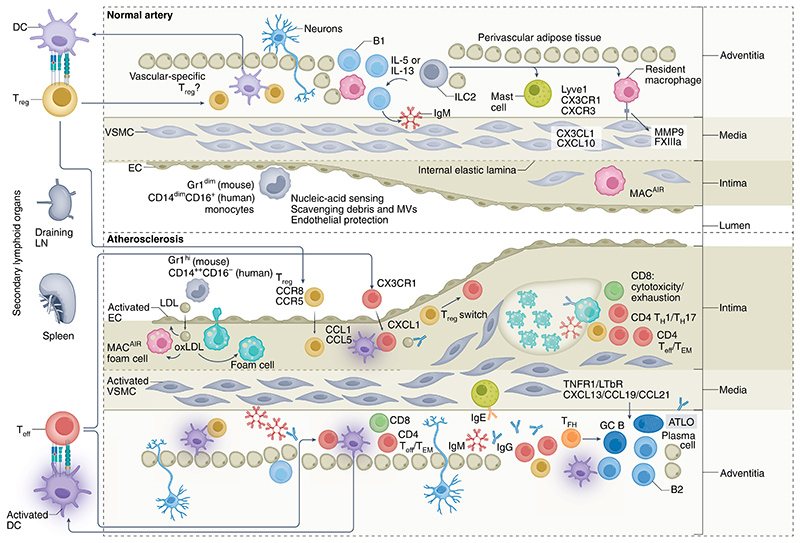
Fig. 1. The immuno-vascular unit in health and atherosclerotic disease.
VALT is present in normal, healthy arteries and comprises a variety of immune cells that serve essential roles in immune surveillance and vascular homeostasis. Crawling monocytes (Gr1dim in mice, CD14dimCD16+ in humans) sense nucleic acids, and those released after EC damage scavenge debris and microvesicles (MVs) and ensure EC protection. Intimal macrophages (MACAIR) accumulate at sites of intimal thickening (upper right, normal artery) and are the first myeloid cells that scavenge oxLDL in the initial stages of atherosclerosis (middle left, atherosclerotic plaque). This further drives vascular inflammation and leads to the recruitment of inflammatory monocytes (Gr1hi in mice; CD14++CD16− in humans) that give rise to a variety of macrophage subsets. Adventitial macrophages, particularly Lyve1+ macrophages (upper right, normal artery), regulate arterial stiffness, in part through the production of MMP9 and FXIIIA. Their specific contribution to atherosclerosis is currently unknown. The adventitia and perivascular adipose tissue form a permissive niche for the accumulation of atheroprotective ILC2 and innate-like B cells, organized in FALCs (upper middle, normal artery). ILC2-derived type 2 cytokines (for example, IL-5 and IL-13) promote B1 cell activation and the production of natural IgM antibodies, and contribute to an anti-inflammatory macrophage phenotype. Most DCs and T cells of normal arteries are found in the adventitia. T cells of normal arteries are likely to be enriched for a regulatory phenotype146, instructed by DCs presenting vascular-associated antigens (in draining lymphoid organs) and maintaining peripheral tolerance. The inflammatory milieu of developing lesions promotes DC maturation, which favors the generation of TH cells, Teff cells and TEM cells (mostly in draining lymphoid organs) and their recruitment into both the intima and adventitia of atherosclerotic arteries (bottom left). Sustained stimulation of autoreactive Treg cells may downregulate Foxp3, promoting their conversion into TH1 cells and Teff cells. Some of the Treg cells and Teff cells acquire a resident memory phenotype. TH1 cells, Teff cells and TEM cells predominate in advanced lesions (bottom right), and activated cytotoxic CD8+ T cells may acquire an exhausted phenotype. GC activation in secondary lymphoid organs leads to the production of affinity-matured class-switched (IgG, IgE) antibodies, which accumulate in lesions. Medial VSMCs of advanced lesions produce CXCL13 and CCL21 and may adopt features of lymphoid tissue organizer-like cells, leading to ATLO formation (bottom right). ATLOs are conducive to the generation of Treg cells, which serve a counter-regulatory, atheroprotective role. The diseased adventitia also establishes neuro–immune vascular interactions, which affect lesion progression.