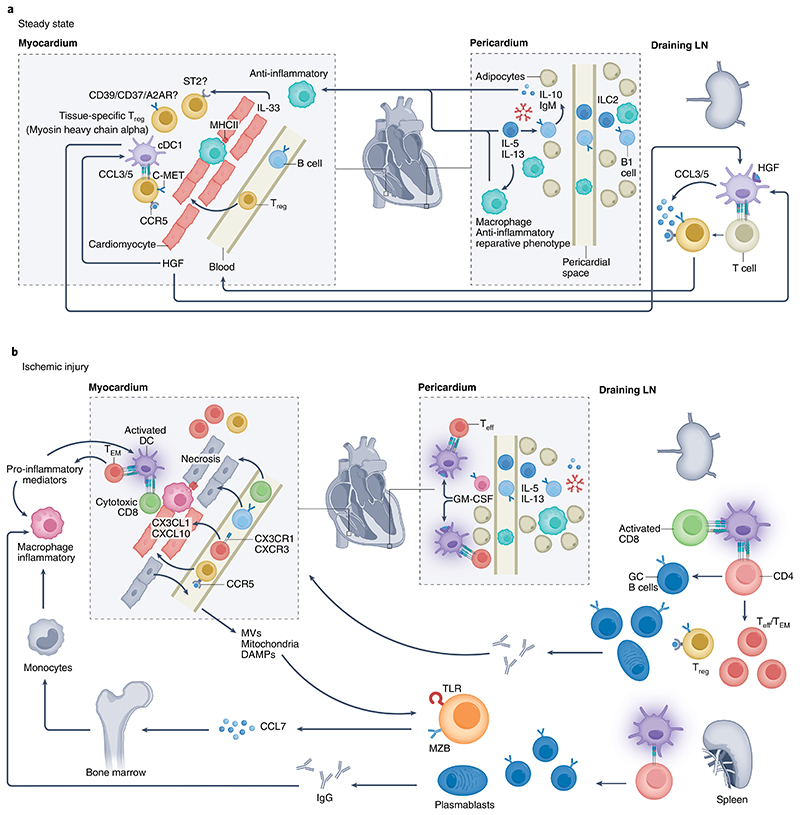
Fig. 2. Adaptive immune responses in the healthy and ischemic heart.
The heart and its adjacent pericardial and adipose tissue harbor several types of immune cells. a, At steady state, conventional DCs (cDCs), particularly cDC1, home to draining LNs and instruct the generation of tissue-specific (for example, myosin heavy chain-α) Treg cells146, which establish peripheral tolerance. Heart-derived HGF binds to DCs and induces an immune-regulatory phenotype. HGF also promotes chemokine production (for example, of CCL5) by c-MET-expressing T cells, which drives CCR5-dependent recruitment and cardiac Treg cell accumulation. Treg cell immunosuppressive function is further promoted through a local (cardiac) paracrine/autocrine adenosinergic loop, involving CD39/CD73 and adenosine A2A receptor (A2AR), or by engaging ST2 signaling with heart-derived IL-33. Pericardial innate lymphoid cells type 2 (ILC2)-derived IL-5 and IL-13 activate innate-like B cells to produce natural IgM antibodies and anti-inflammatory IL-10, which contribute to an anti-inflammatory and reparative macrophage phenotype. b, During ischemic injury, DCs acquire an inflammatory phenotype that provides instructions for the development of TH cells, Teff cells and TEM cells, as well as CD8+ cytotoxic T cells. Enhanced production of GM-CSF by pericardial innate-like B cells further promotes DC activation and the generation of TH cells and Teff cells. These T cells upregulate CX3CR1 and CXCR3, and their recruitment into the ischemic heart is facilitated by heart-derived CX3CL1 and CXCL10. GC reactions develop in the spleen and draining LNs, leading to the production of (heart-specific) autoantibodies, with potential detrimental consequences. Splenic MZ B cells are activated by the release of DAMPs, including in the form of mitochondria-containing microvesicles, which activate Toll-like receptors (TLRs), leading to CCL7 production. The latter promotes inflammatory monocyte mobilization from the bone marrow, enhancing the accumulation of inflammatory macrophages within the ischemic myocardium. In most cases, the ischemic heart is able to maintain the presence of a sufficient number of Treg cells to prevent the occurrence of overt full-blown cardiac autoimmunity.