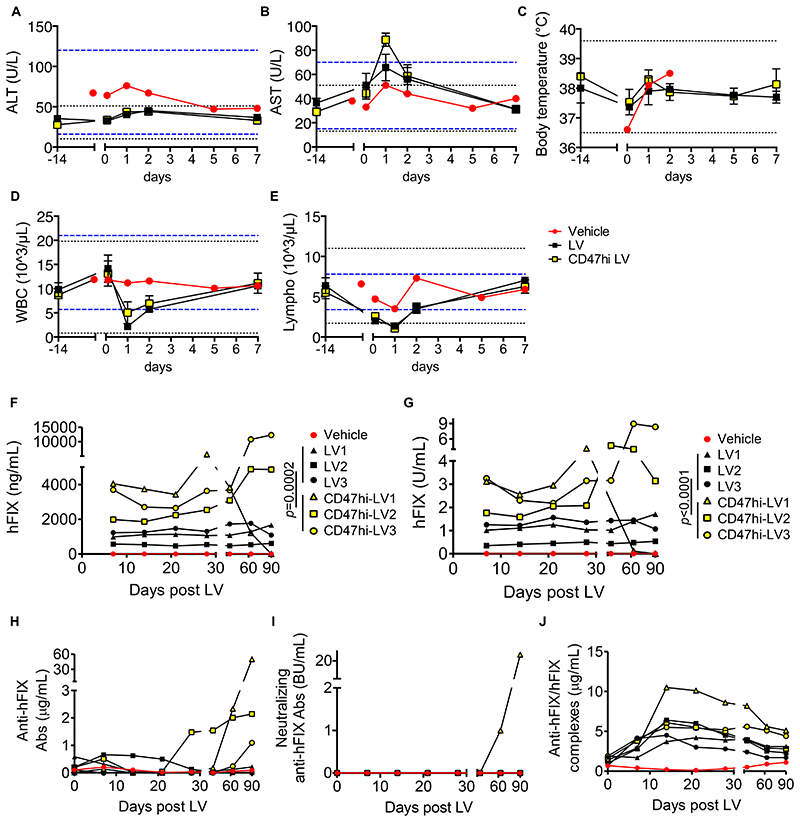
Fig. 4. Tolerability and efficacy of i.v. LV gene therapy in NHP.
(A-E) Mean with SEM of the concentration of (A) ALT, (B) AST, (C) body temperature, (D) counts of WBC and (E) lymphocytes of vehicle (n=1, red circles), LV-treated (n=3, black squares) or CD47hi-LV treated NHP (n=3, yellow squares) at the indicated time after administration. The black dashed lines show the mean±3SD calculated on 14 pre-LV samples taken from the same animals; the blue dashed lines show the normal reference values for Macaca fascicularis. (F-J) Concentration of (F) human FIX antigen or (G) human FIX activity measured in the plasma, or (H) total anti-human FIX Abs, or (I) neutralizing anti-human FIX Abs, or (J) anti-human FIX/human FIX immune complexes measured in the serum of vehicle (n=1, red circles), LV-treated (n=3, black symbols) or CD47hi-LV treated NHP (n=3, yellow symbols) at the indicated time after administration; U: units; BU: Bethesda Units. Non-parametric two-way ANOVA on the first 30 days post LV.