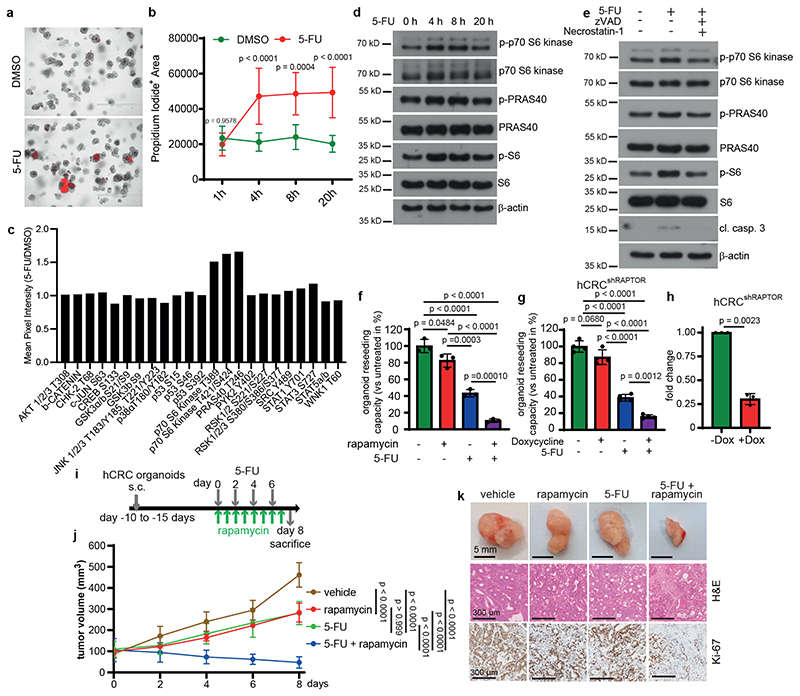
Figure 1. Resistance of colorectal patient-derived tumor organoids to 5-FU depends on mTOR activation.
a, Propidium iodide (PI) staining of hCRC organoids treated with 5-FU for 4h (n = 3 biological replicates, scale bar = 1000 μm).
b, Quantification of PI+ area of 5-FU treated hCRC organoids (1h and 8h DMSO: n=6, 4h
DMSO: n=9, 20h DMSO: n=8h, 1h and 8h 5-FU: n=7, 4h and 20h 5-FU n=10, data were pooled from 3 biological replicates).
c, Kinase activation assay of 20h 5-FU treated hCRC organoids. Results represent fold increase of pixel intensity (5-FU/DMSO, n=1). For corresponding dot blot see SI.
d, Immunoblotting of hCRC organoids treated as indicated (n=2).
e, Immunoblotting of hCRC organoids, treated with zVAD and Necrostatin-1 2h prior to 4h 5-
FU treatment (n=2).
f, Reseeding capacity of hCRC organoids treated as indicated (n=3, one of four biological replicates).
g, Reseeding capacity of doxycycline-inducible hCRCshRAPTOR organoids treated as indicated (n=4, one of three biological replicates).
h, Raptor qRT-PCR analysis of doxycycline treated hCRCshRAPTOR tumor organoids (n=3 biological replicates).
i, Treatment regimen of subcutaneous hCRC tumors.
j, Growth rate of tumors in response to the treatment indicated in (i). n=6 mice for each condition. One set of mice was analysed.
k, Representative images of subcutaneous hCRC tumors (scale bar = 5mm) and H&E and Ki-67 staining (scale bar = 300 μm) of tumor tissues of mice treated with 5-FU +/- rapamycin as indicated in (j), (n=6, staining was performed on all tumors indicated in (j)).
All data are mean ±SD and analysed by two-tailed Student’s t-test (h) or 1-way ANOVA (f, g) and 2-way ANOVA (b, j), with Bonferroni’s multiple comparison.