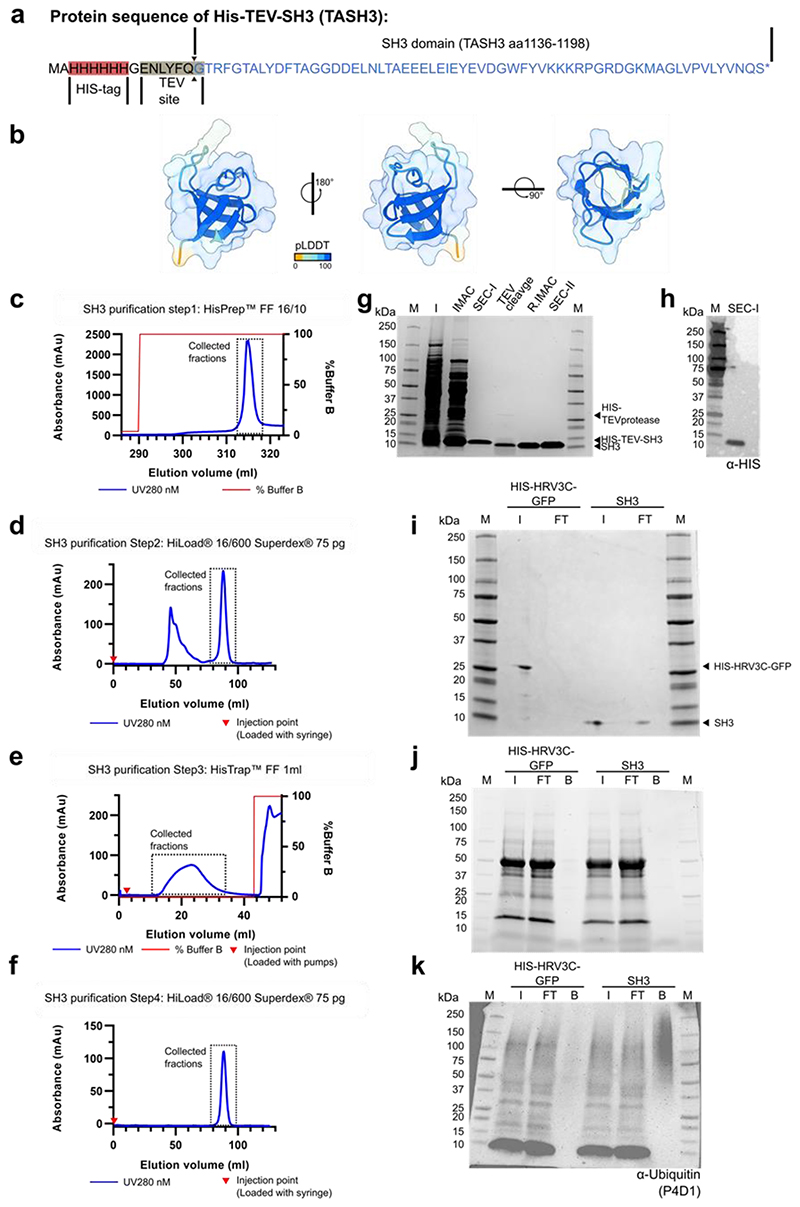
Extended Data Fig. 7. Recombinant TASH3_SH3 domain purification.
a) Protein sequence of the construct used for recombinant TASH3_SH3 production. b) Predicted structural model of amino acids 1136-1198 of TASH3 by Alphafold2. c-f) Purification of recombinant HIS-TEV-SH3 domain (TASH3). The collected fractions are indicated by a dotted line. c) Immobilized metal affinity chromatogram of the first step in the purification. d) Size exclusion chromatogram of the second step in the purification. The fractions collected in the first step were loaded on a HiLoad® 16/600 superdex®75pg column. e) Reverse Immobilized metal affinity chromatogram of the third step in the purification. HIS-TEV-SH3 collected in the second step was subjected to overnight TEV-cleavage with HIS-TEV protease and loaded on a HisprepTM Fast Flow 1 ml column (HIS-TEV was removed). f) Size exclusion chromatogram of the final step in the purification. The fractions collected in the third step were loaded on a HiLoad® 16/600 superdex®75pg column. g) Coomassie-stained SDS-PAGE gel of the different steps of the TASH3_SH3 domain purification process. IMAC – collected fractions from c, SEC-I – collected fractions from d, TEV cleavage - the cleavage of the HIS-TEV from the recombinant protein using a HIS-TEV protease, R.IMAC - the collected fractions from e, SEC-II – the collected fractions from f. HIS-TEV-SH3: 8.8kDa, SH3: 7.0kDa, HIS-TEV protease: 28kDa. h) Western blot detection using an anti-5xHIS antibody on the collected fractions of the second step of purification (SEC-II), showing that the collected fractions contain HIS-TEV-SH3. i) Coomassie-stained SDS-PAGE gel showing the coupling efficiency of HIS-HRV3C-GFP and TASH3_SH3 on PierceTM NHS-Activated agarose beads. Covalent coupling of the recombinant protein to the beads can be observed as a reduction in the intensity in the flow-through. j) Stain-freeTM SDS-PAGE gel showing Col-0 extracts which were incubated with the HIS-HRV3C-GFP or TASH3_SH3 coupled beads. k) Western blot detection using a general anti-Ubiquitin antibody (P4D1) showing Col-0 extracts which were incubated with the HIS-HRV3C-GFP or TASH3_SH3 coupled beads. The smear in the bound fraction indicates that the SH3 domain from TASH3 can bind ubiquitinated proteins as opposed to the HIS-HRV3C-GFP control. I – Input, FT - flow-through, B – bound fractions.