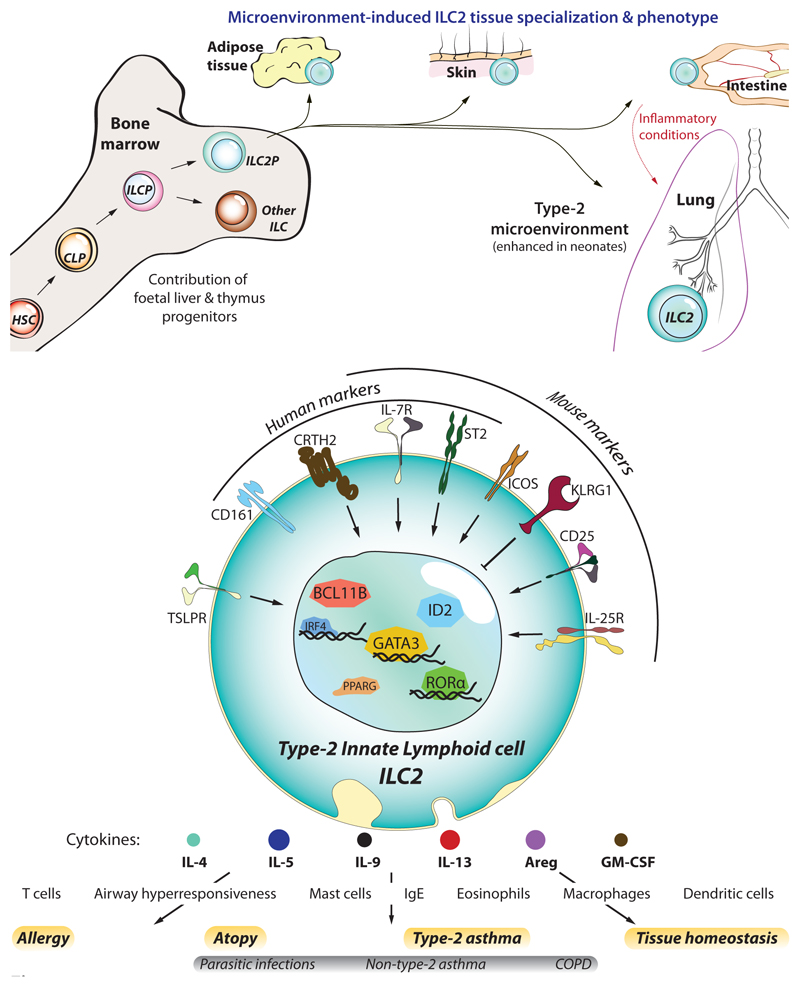
Figure 1. ILC2 development and identification.
(a) Like other haematopoietic cells, ILC2s derive from haematopoietic stem cells (HSC) that differentiate into common lymphoid progenitors (CLP), and further commit towards the innate lymphocyte lineage as ILC progenitors (ILCP), and ILC2 progenitors (ILC2P). Though ILC2s first develop in foetal tissues, a significant fraction of them arise during the neonatal period whereupon they colonise most peripheral tissues (skin, intestine, lung, and visceral adipose tissue – VAT). Here they becom resident lymphocytes and adapt to the tissue microenvironment by acquiring a specialized genetic program tailored to each tissue. In particular, at least half of the ILC2s residing in the lung are from neonatal origin, a period where lungs display a strong type-2-biased microenvironment and allergic sensitisation likely occurs. However, under inflammatory conditions such as parasite infection or allergic reactions, the lung receives a transient ingress of ILC2s from the small intestine, which are characterized by increased sensitivity to IL-25 and higher expression of KLRG1, that contribute to lung inflammation. (b) ILC2s can be identified by their lack of lineage specific markers (CD3, CD4, CD8a, CD11b, CD11c, CD19, FcεRI, Ly6C, Ly6G, NK1.1/CD56, TCRαβ, TCRγδ, and Ter119), and expression of IL-7Rα (CD127) and high levels of the transcription factor GATA3. In addition, other molecules can serve to identify them in humans (i.e. CD161, CRTH2, and ST2) and mice (ST2, CD25, KLRG1, ICOS). Despite also providing contact-dependent signals, the foremost contribution of ILC2s to allergy and asthma is their overwhelming capacity to rapidly produce type-2 cytokines upon stimulation by cytokines/alarmins (IL-25, IL33, TSLP). In turn, ILC2-derived cytokines orchestrate the spectrum of immune effector cells that mediate the immunopathology of asthma, such as eosinophils and mast cells.