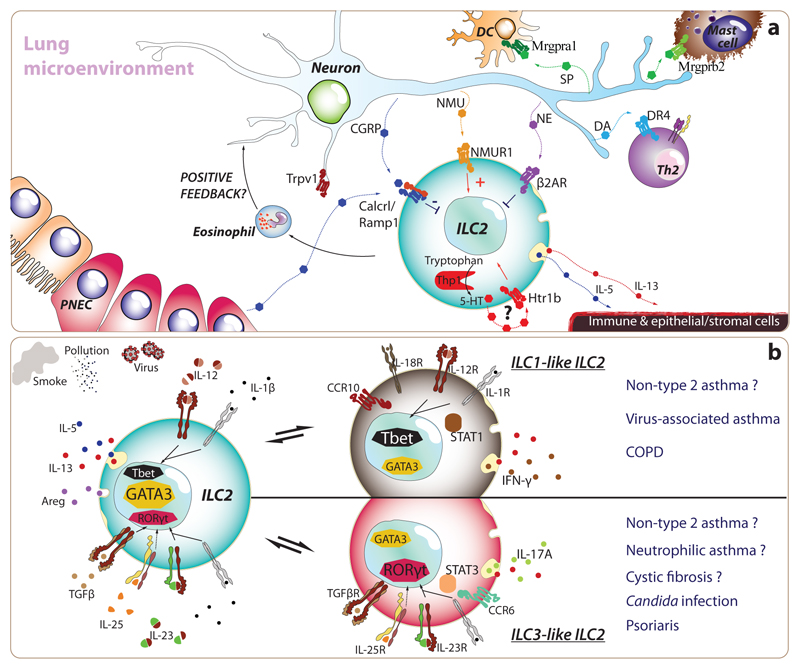
Figure 4. Environmental and microenvironmental cues leading to typical and atypical ILC2s in asthma.
(a) Recently, researchers have discovered that ILC2s express many receptors for neurotransmitters and neuropeptides, as well as exceptionally high levels of certain enzymes implicated in the synthesis of neurotransmitters. Both sympathetic and vagal sensory neurons project their afferent fibres within lung parenchyma. In particular, transient-receptor-potential-cation channel-subfamily-V-member-1-expressing nociceptive neurons (Trpv1+), which specialize in the detection of noxious stimuli from the environment (e.g. irritants, extreme temperatures, tissue damage), appear to play key roles in promoting airway hyperresponsiveness. Besides activating type-2 classical dendritic cells (cDC2) and mast cells through the production of Substance P (SP), and promoting type-2 T helper cell differentiation through the release of dopamine, lung afferent neurons release many peptides and biogenic amines which directly target ILC2s, which reside in close proximity of neural fibres. Neuromedin U (NMU) promotes ILC2 expansion and Th2 cytokine production while norepinephrine (NE) and calcitonin gene related peptide (CGRP) curb the activation of ILC2s. Furthermore, unusually for an immune cell, ILC2s can secrete CGRP and express the enzymatic machinery necessary to produce serotonin (5-TH). Murine models have shown that inhibition or deletion of nociceptive neurons prevents or decreases the severity of allergic immune responses and asthma, suggesting that modulation of neuro-immune units can represent a targetable treatment approach. (b) ILC2s are characterized by the expression of cardinal type-2 cytokines (i.e. IL-4, IL-5, IL-9, IL-13 and Areg) and the transcription factor, GATA3. Nonetheless, ILC2s exhibit a remarkable ability to adapt their biology to match that of their environment. This reversible plasticity allows ILC2s to acquire ILC1- and ILC3-like features such as specific expression of Tbet and RORγt and IFN-γ and IL-17A, respectively. Indeed, the changes induced by IL-1β and IL-12, in the case of ILC1-like ILC2s, or by IL-1β, IL-23 and TGFβ for ILC3-like ILC2s suggest a profound modulation in the transcriptional signature. Both human and murine ILC2s present this capacity in vitro, but we are only starting to understand the prevalence of this process in vivo. Interestingly, the plasticity of ILC2s provides a plausible hypothesis to understand how ILC2s may participate and promote COPD and non-type-2 asthma that are characterised by type-1 and type-3 immunity respectively.