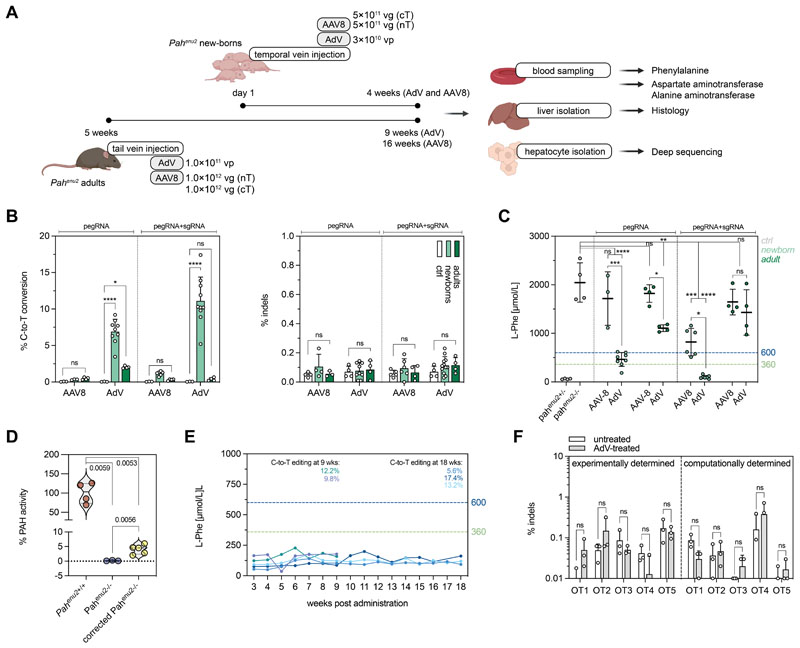
Figure 4. Correction of the Pahenu2 allele in vivo in mice using prime editing.
(A) Schematic outline of the experimental setup for AAV8- and AdV-mediated treatment in newborn and adult PKU mice (vg, viral genomes; vp, viral particles). (B) In vivo correction and indel rates in newborn and adult animals after AAV8- or AdV-mediated delivery of SpCas-PEs (injected dose of AAV8 in neonates and adults: 1×1012 and 2×1012 vg; injected dose of AdV in neonates and adults: 3.0×1010 and 1.0×1011 vp). Untreated mice were used as negative controls. Indels are calculated as percentage of sequencing reads with indels at the protospacer region. (C) Blood L-Phe concentrations after in vivo prime editing compared to untreated, heterozygous, and homozygous control animals. L-Phe concentrations below 600 μmol/L (U.S.) and 360 μmol/L (Europe) are considered therapeutically satisfactory (61, 62). (D) Enzymatic activity of PAH at experimental endpoints (4 weeks, AdV). (E) Blood L-Phe concentrations in newborn AdV-treated mice over time. Editing rates of the corresponding mice are color-coded and indicated at 9 and 18 weeks. (F) Deep amplicon sequencing of the top 5 experimentally determined off-target (OT) sites for the protospacer of the pegRNA mPKU-2.1 and of the top 5 computationally predicted off-target sites in untreated and AdV-treated mice (>20’000 reads per site). Data are represented as mean ± s.d. (n=3-8 mice per group) and were analyzed using a two-way ANOVA with Tukey’s multiple comparisons test (ns, not significant, P>0.05; *P<0.05; **P<0.005; ***P<0.0005; ****P<0.0001).