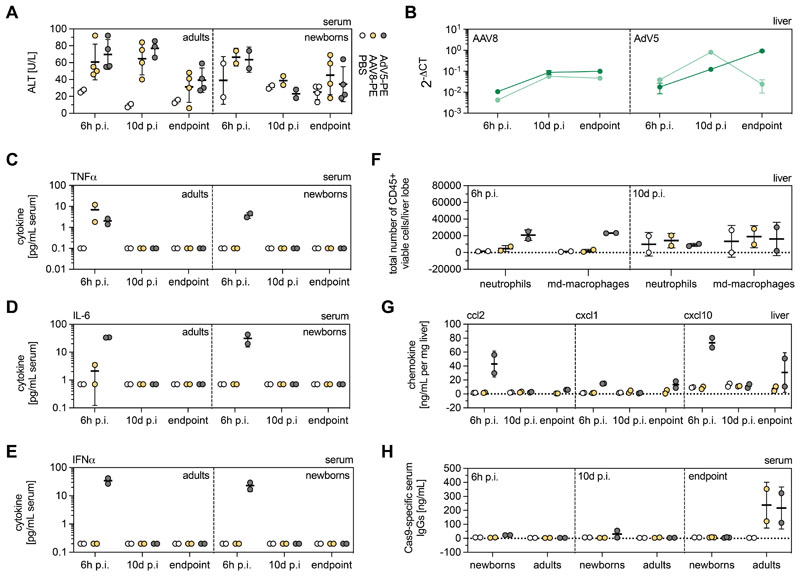
Figure 5. Innate and adaptive immunity to AAV8 and AdV vectors.
(A) ALT concentrations in PBS-, AAV8- and AdV-treated mice at 6h p.i., 10d p.i., and experimental endpoints. (B) Cas9 transcript abundance in AAV8- and AdV-treated newborns (light green) and adults (dark green) at 6h p.i., 10d p.i., and endpoints. Transcript counts were normalized to the housekeeping gene Rplp0. (C-E) TNFα (C), IL-6 (D), and IFNα (E) concentrations in the serum of PBS-, AAV8- and AdV-treated adults and neonates at 6h, 10d p.i. and experimental endpoints. (F, G) Presence of neutrophils and monocyte-derived (md) macrophages (F) and pro-inflammatory chemokines ccl2, cxcl1, and cxcl10 (G) in the livers of adult mice at 6h p.i. and 10d p.i. (H) Cas9-specific adaptive immune response in control and treated mice 6h, 10d p.i. and endpoints. Data are represented as mean ± s.d (n=2-4 animals per group). Experimental endpoints for AdV, 4 weeks (newborns and adults); for AAV8, 4 weeks (newborns), 8 weeks (adults).