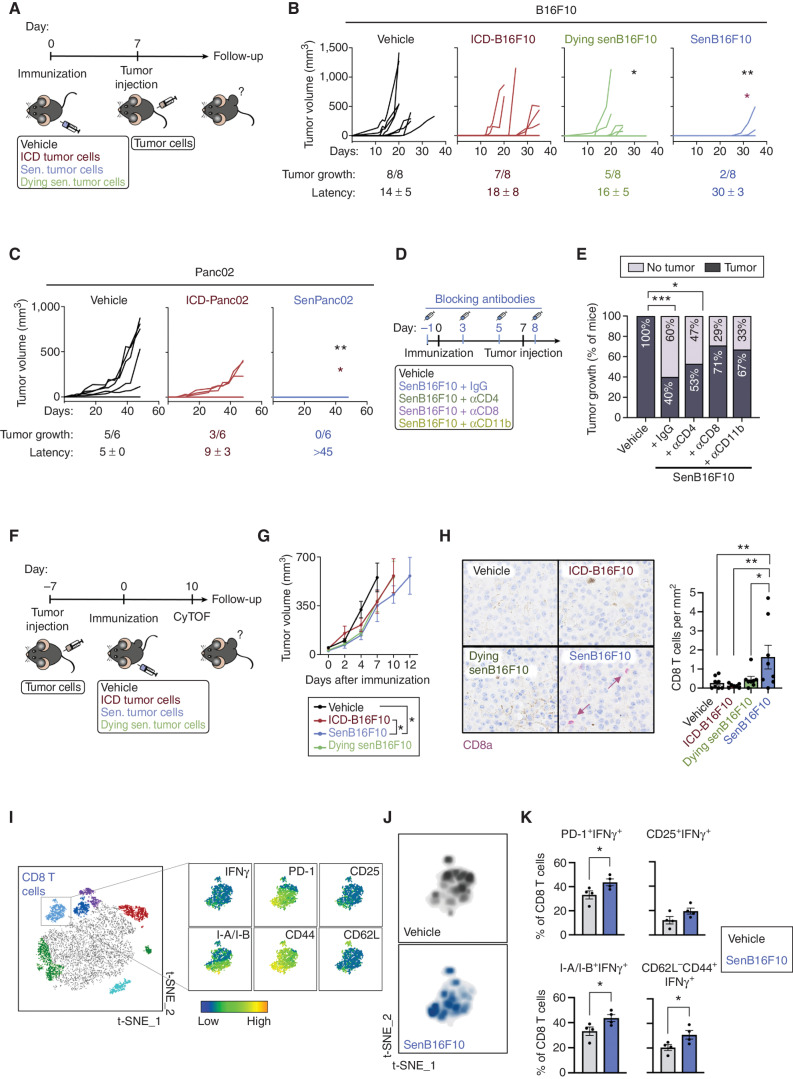
Figure 4.
Immunization with senescent cancer cells promotes anticancer immune surveillance. A, Schematics of the cancer immunization protocol used in these studies. Sen., senescent. B, Individual tumor growth curves from vehicle-treated mice or mice immunized with ICD-B16F10, senB16F10, or senB16F10 cells dying by senolysis (induced by 10 μmol/L navitoclax, dying senB16F10; n = 8 mice per group). Tumor growth (number of animals developing tumors out of the total) and tumor latency (mean ± SD of the day on which the tumor appeared) are indicated for each group. **, P < 0.01; *, P < 0.05; two-way ANOVA test compared with vehicle-treated group (black) or ICD-B16F10 group (red). C, Individual tumor growth curves from vehicle-treated mice or mice immunized with Panc02 cells dying by ICD (induced by a high dose of doxorubicin, ICD-Panc02) or senescent Panc02 (low dose of doxorubicin, senPanc02; n = 6 mice per group). Tumor growth (number of animals developing tumors out of the total) and tumor latency (mean ± SD day of appearance of the tumor) are indicated for each group. **, P < 0.01; *, P < 0.05; two-way ANOVA test compared with vehicle-treated (black) group or ICD-B16F10 group (red). D, Schematics of the cancer immunization and immune depletion protocol used in this study. E, Tumor appearance after rechallenge in vehicle-treated mice (n = 14) or mice immunized with senB16F10 treated with IgG (n = 14) or the indicated blocking antibodies as described in D (n = 15 for aCD4, n = 14 for aCD8, or n = 6 for aCD11b). ***, P < 0.001; *, P < 0.05; Fisher exact test. F, Schematics of the therapeutic cancer immunization protocol used in these studies. CyTOF, cytometry by time of flight. G, Grouped tumor growth of B16F10 tumor–bearing animals immunized with vehicle, ICD-B16F10, dying senB16F10, or senB16F10. *, P < 0.05; two-way ANOVA test (n = 7–8) H, CD8a staining (purple) in B16F10 tumor sections from animals immunized with vehicle, ICD-B16F10, dying senB16F10, or senB16F10 and sacrificed at humane endpoint. Note that the brown pigmentation is due to melanin. Representative images and quantification of n = 7–8 mice per group. **, P < 0.01; *, P < 0.05; one-way ANOVA test. I, t-Distributed stochastic neighbor embedding (t-SNE) representation of tumor-infiltrating immune (CD45+) cells detected by CyTOF of B16F10 tumors from animals immunized with vehicle or senB16F10 and sacrificed 10 days after immunization. The cluster of CD8 T cells is amplified, and the expression pattern of T-cell markers of activation (IFNγ, PD-1, CD25, and I-A/I-B), and differentiation to effector (CD44) or naïve T cells (CD62L) are shown (left). J, Density plots of the distribution of infiltrating CD8 T cells from nonimmunized and senB16F10-immunized animals (n = 4 animals per group). K, Percentage of activated tumor-infiltrating CD8 T cells (PD-1+IFNγ+, CD25+IFNγ+, I-A/I-B+IFNγ+, CD62L−CD44+IFNγ+) from tumors of nonimmunized animals (vehicle) or immunized with senB16F10 (n = 4 mice per group). *, P < 0.05; unpaired Student test.