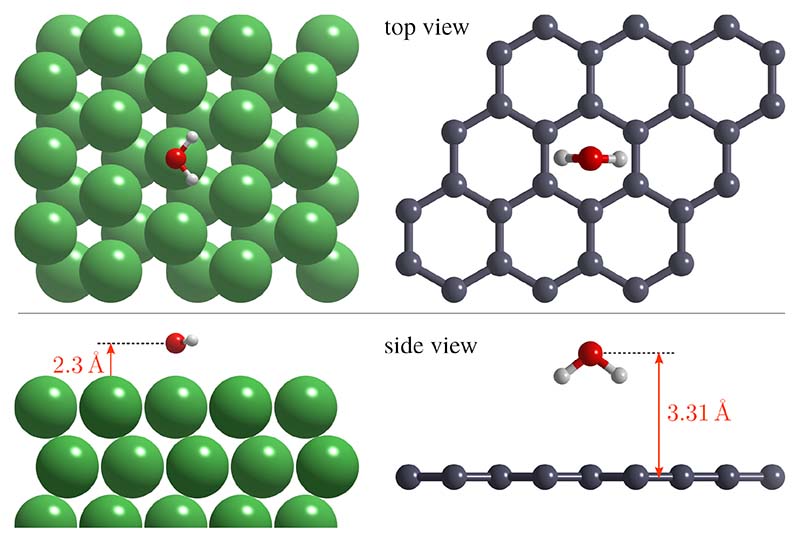
Figure 4.
Comparison showing the radically different adsorption geometries for a single water molecule on a metallic surface and graphene. On a close-packed metal surface such as Ru(0001), H2O adsorbs with the oxygen atom on top, yielding a higher binding energy and a vertical orientation of the molecule[68], in contrast to the most favourable adsorption geometry on graphene from dispersion corrected DFT[56] with H2O in the centre of the hexagon and both O–H bonds pointing towards the surface.