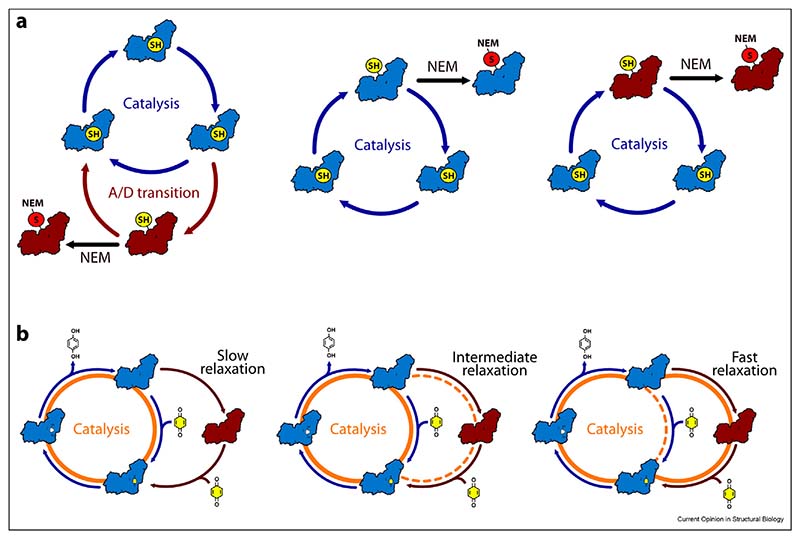
Scheme 2. Developing a unified explanation for the behaviour of slow-relaxing and fast-relaxing variants of complex I.
Closed states are shown in blue and open states in red. a) Simple conceptual possibilities for the status of the ND3-TMH1–2 loop and Cys39. Cys39 (SH, yellow) can be occluded, as in the closed/active resting enzyme, or exposed, as in the open/deactive resting enzyme. When exposed it is available for derivatisation by NEM (S-NEM, red). Left: Cys39 is only exposed and derivatised following relaxation into an off-cycle open/deactive state. Middle: Closed states transiently expose Cys39 during turnover allowing it to be derivatised. Right: Open states formed during catalysis expose Cys39 for derivatisation by NEM. b) A slow-relaxing enzyme (left, for example wild-type mouse complex I) catalyses using only closed states; the resting active/closed enzyme relaxes slowly into the deactive/open state, which reactivates slowly upon addition of substrates. In contrast, a fast-relaxing enzyme (right, for example the ND6-P25L mouse variant) most often follows the detour cycle, through an open, deactive-like state, in which enzyme opening and closing both occur on the catalytic timescale. Intermediate cases (the porcine, bovine and ovine enzymes) may follow either pathway, depending on their relative rates of relaxation and onward catalysis (middle). Catalytic pathways are highlighted in orange, with dashed lines indicating less favoured routes. ubiquinone and ubiquinol are shown in yellow and white, respectively.