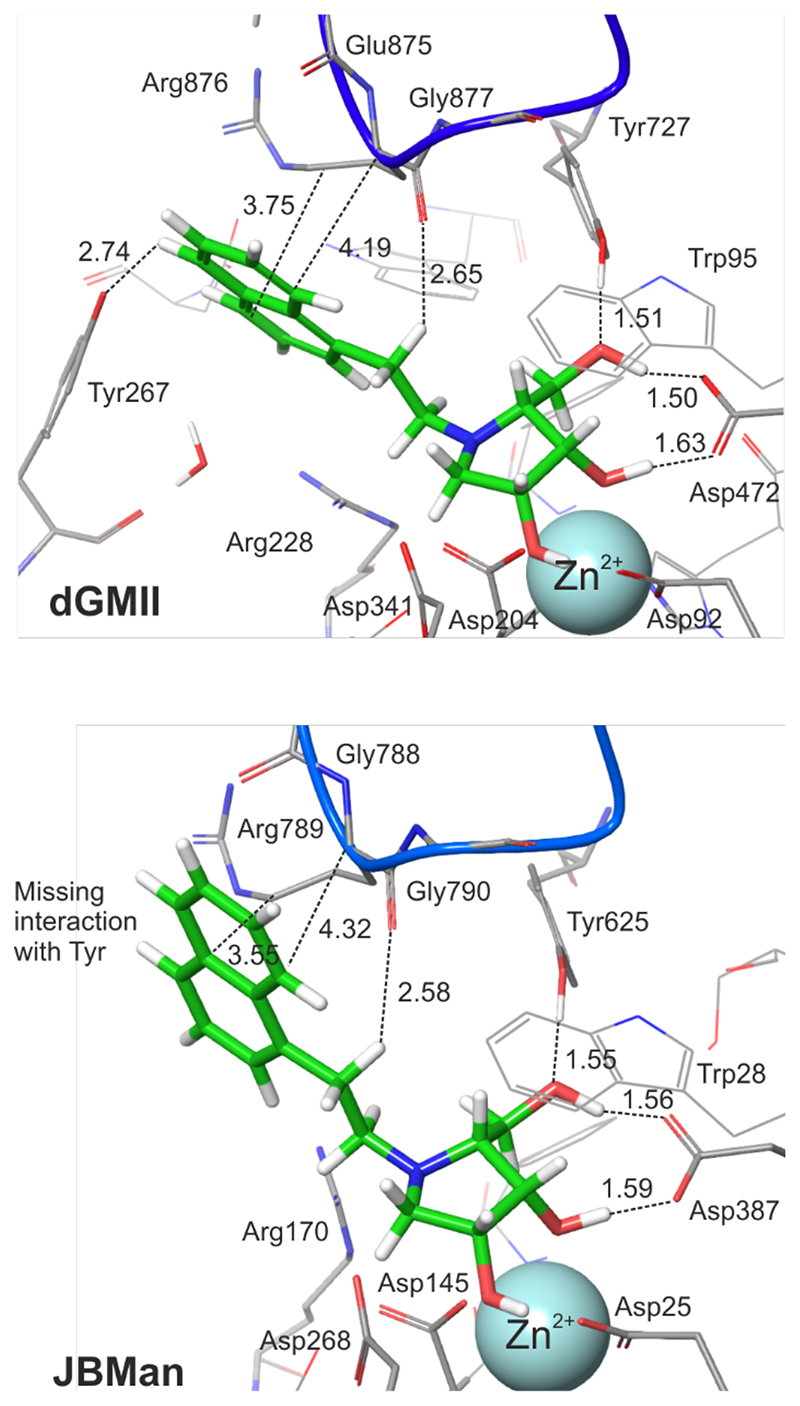
Figure 4. QM/MM optimized structures of the complexes 18:dGMII and 18:JBMan.
For sake of clarity most hydrogen atoms are not visualized. Some hydrogen bonds as well as contact interactions of the naphtyl linker with a loop (in blue color, the Glu875-Arg876-Gly877 sequence in dGMII and Arg789-Gly788-Gly790 in JBMan) are shown by dash lines (values of distances in Å). Interactions between the naphtyl linker of the inhibitor and Tyr267 in dGMII is missing in JBMan where the active site is more open and solvent accessible.