Figure 1.
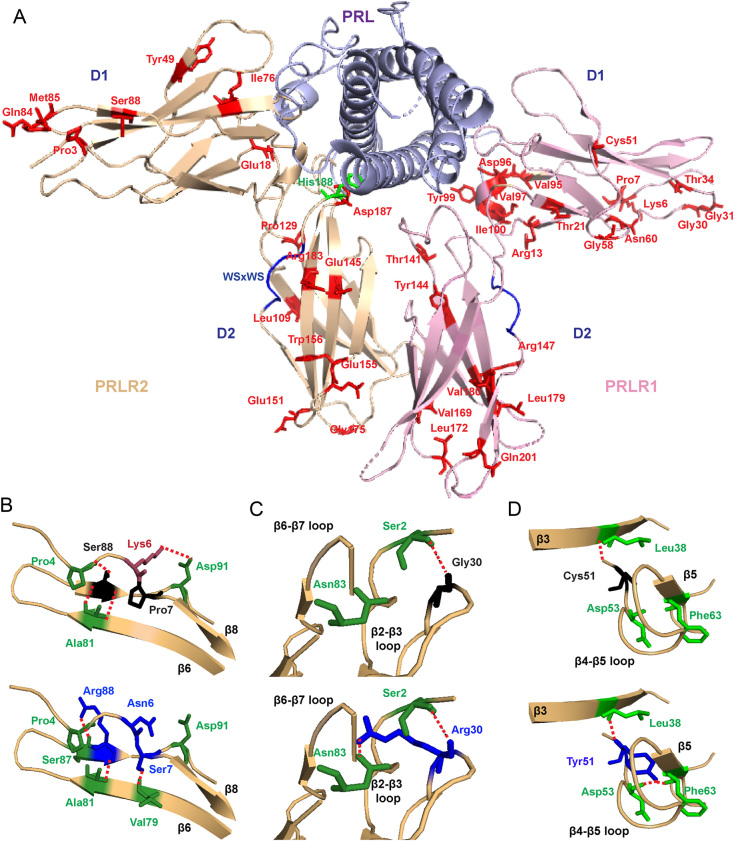
Location of the PRLR ECD variants and structural characterisation of rare variants located in D1. (A) Crystal structure showing the two monomers (PRLR1 in pink and PRLR2 in light brown) of the rat PRLR extracellular domain (ECD) in complex with the PRL hormone (purple) (PDB 3NPZ (van Agthoven et al. 2010)). Each PRLR ECD monomer is comprised of two subdomains, designated D1 (residues 1–101) and D2 (residues 109–210), which are important in ligand binding and subsequent receptor activation (Svensson et al. 2008, Broutin et al. 2010, van Agthoven et al. 2010, Rao and Brooks 2011, Brooks 2012). Side chains of the potential deleterious variant residues from ExAC and GnomAD are shown in red. The His188 residue that is mutated in hyperprolactinaemia is shown in green. Residue 49 is labelled as Tyr in the rat structure, and in humans, this is His49. Residue 169 is labelled as Val in the rat structure, and in human, this is Ile169. Rare variants are shown in only one monomer. (B) Lys6, Pro7, and Ser88 are located in close proximity within the β6-β7-β8 region (Top). The variant Asn6 loses contact with Asp91 on the β8 strand, Ser7 gains contact with Val79 on the β6 strand, and Arg88 loses contact with Pro4 and gains a new contact with the adjacent Ser87 (all blue, bottom). (C) The Gly30 residue, located in the β2-β3 loop, forms a polar contact with the Ser2 residue (Top). When mutated to Arg30 (blue, bottom), the longer side chain is able to retain this contact and form a new hydrogen bond with Asn83 in the β6-β7 loop. (D) The Cys51 residue is located in the β4-β5 loop. Mutation in Tyr51 (blue, bottom) adds a more bulky residue within this tightly packed region, which is predicted to result in gain of two contacts with Asp53 and Phe63 of the β4-β5 loop.

 This work is licensed under a
This work is licensed under a