Figure 3.
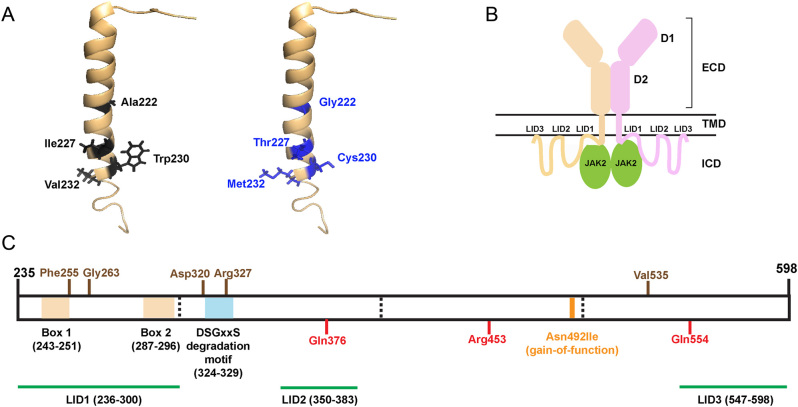
Structural characterisation of the PRLR variants located in the TMD and ICD. (A) A single α-helix forms the transmembrane domain (TMD) of the PRLR. Five rare PRLR variants (Ala222Gly, Ile227Thr, Trp230Cys, Val232Ala, Val232Met) are located within the TMD, and four of these are present within the published NMR structure of the PRLR TMD (PDB:2N7I (Bugge et al. 2016)). The four WT residues (black) are located at the cytoplasmic end of the TMD and each forms backbone contacts with adjacent residues within the α-helix. The mutant residues (blue) are not predicted to affect these backbone contacts. (B) Cartoon depicting the PRLR structure with the two monomers shown in brown and pink. The extracellular domain (ECD) contains two domains (D1 and D2) and is connected to the intracellular domain (ICD) via the TMD. The ICD is predicted to interact with the plasma membrane in at least three regions known as lipid-interacting domains (LID1-3). The ICD also interacts with the JAK2 proteins that activate signalling downstream of the PRLR. (C) Cartoon showing the known functional domains of the PRLR ICD, with the amino acid residues (236–300, 243–251, 324–329, 350–383, and 547–598) involved shown in parentheses. The LIDs are shown in green. Two regions, Box 1 and Box 2 (brown-shaded regions), are binding sites for JAK2 (Lebrun et al. 1995), and the DSGxxS region (blue-shaded region) acts as a degradation motif (Plotnikov et al. 2009). The PRLR residues investigated in this study are shown in brown above the cartoon and residues investigated in previous studies are shown in red below the cartoon (Bernard et al. 2016, Gorvin et al. 2018a). The location of the Asn492Ile gain-of-function PRLR variant that is associated with prolactinoma is indicated in orange.

 This work is licensed under a
This work is licensed under a