Figure 2. mRNA poly(A) tails stimulate translation.
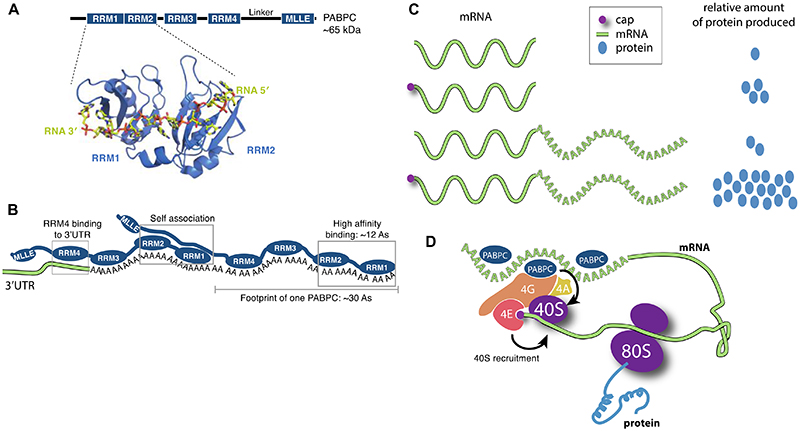
A Structure of eukaryotic poly(A)- binding protein (PABPC – Pab1 in yeast, PABPC1 in mammals). Domain diagram of the conserved PABPC is shown with the position of the four RNA-recognition motif (RRM) domains and the Mademoiselle (MLLE) domain indicated38,39. Below, a crystal structure of RRM1-RRM2 (cartoon) of yeast Pab1 bound to poly(A) RNA (sticks) is shown (PDB 1CVJ)42. B Arrangement of PABPC on RNA. Two PABPC molecules can bind a 60 nt poly(A) tail. RRMs 1 and 2 have the highest affinity and specificity for poly(A) and require ~12 As for high affinity binding40,41. Full-length PABPC footprints ~30 nt and adjacent PABPC molecules interact with each other35,45,191. RRM4 may bind to the 3′-UTR130. C The mRNA 5′-cap (magenta circle) and 3′-poly(A) tail act synergistically to stimulate gene expression in eukaryotes. The relative amount of protein produced from reporter mRNAs with and without 5′ cap and poly(A) tail in plant, animal and yeast cells are depicted9. D Closed-loop model. The eukaryotic translation initiation factor 4E (eIF4E) binds the 5′-cap. eIF4G binds both eIF4E and PABPC, as well as the RNA helicase eIF4A, and this is thought to stimulate recruitment of the small (40S) ribosomal subunit. 40S assembles with a large (60S) ribosomal subunit on a start codon to form a translation-competent 80S ribosome.