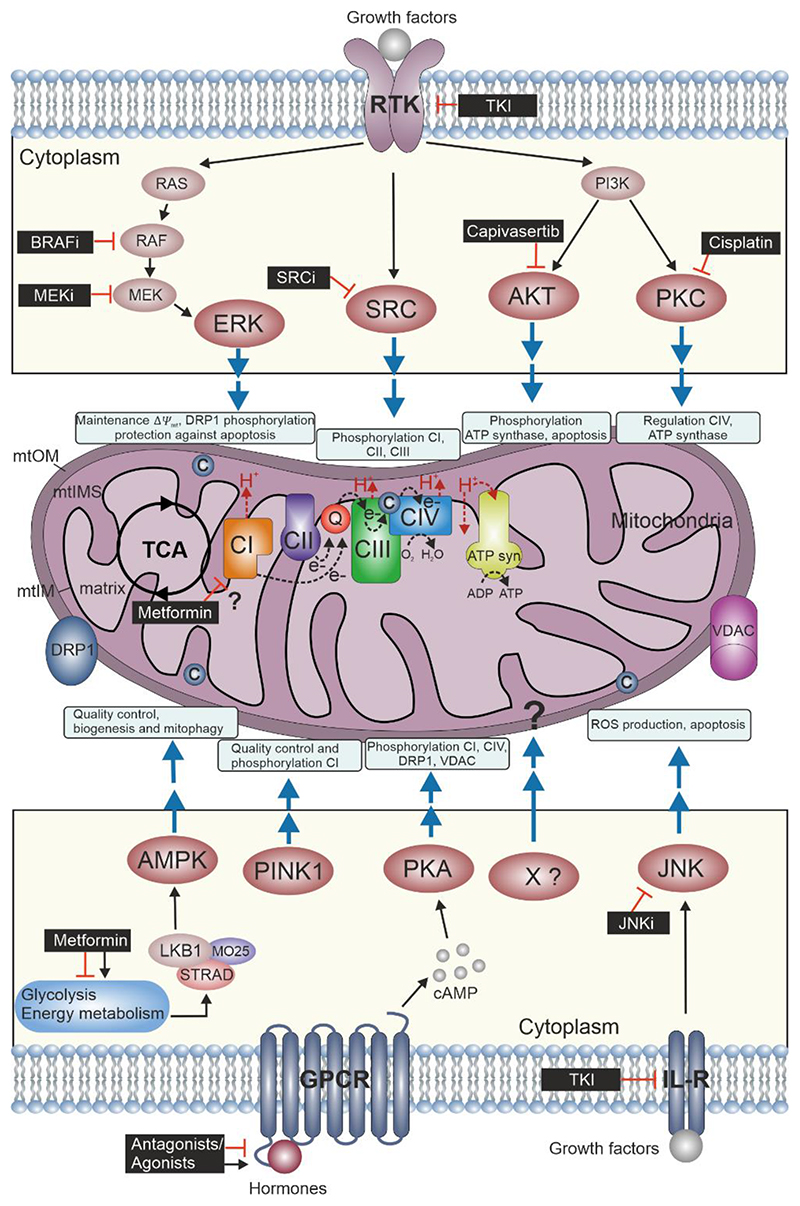
Figure 1. Perturbing the Mitochondrial Kinase Network.
Mitochondria consist of four compartments: outer membrane (mtOM), intermembrane space (mtIMS), inner membrane (mtIM) and matrix. The oxidative phosphorylation (OXPHOS) system comprises the ETS and the phosphorylation system (including ATP synthase [ATP syn]) where the reduced fuel substrates coming from the tricarboxylic acid cycle (TCA) and other metabolic pathways are oxidized by electron transfer, chemiosmotic coupling to the phosphorylation of ADP to ATP and intrinsically uncoupling by proton leak and slip, cation cycling, and electron leak. Electrons fuel from NADH-linked substrates to Complex I (CI) and from succinate to CII. These and other electron transfer pathways converge at the Q-junction with further transfer to CIII, cytochrome c (c) and CIV, where electrons reduce O2 producing H2O. The protonmotive force generated is utilized at the ATP synthase to phosphorylate ADP to ATP (Gnaiger et al 2020). Growth factors bind to RTKs and may activate cell type specifically ERK, SRC, AKT and PKA kinases. These might be involved in directly/indirectly influencing activities of the respiratory complexes or mtOM proteins by phosphorylation and control mitochondrial dynamics and bioenergetics. AMPK, PKA and JNK are activated by diverse types of membrane receptors (i.e. GPCRs and interleukin receptors, IL-R, respectively) and control mitochondrial fission, ETS and apoptosis. AMPK and PINK1 are the mitochondrial rheostat for energy state and quality control in response to cellular stress. Kinase inhibitors (black boxes) modulate mitochondrial function by inhibiting kinase activities. The specificity and efficacy depend on cell type, timing and doses of the applied inhibitors which determine the portfolio of kinases (on-target and off-target) that are affected.