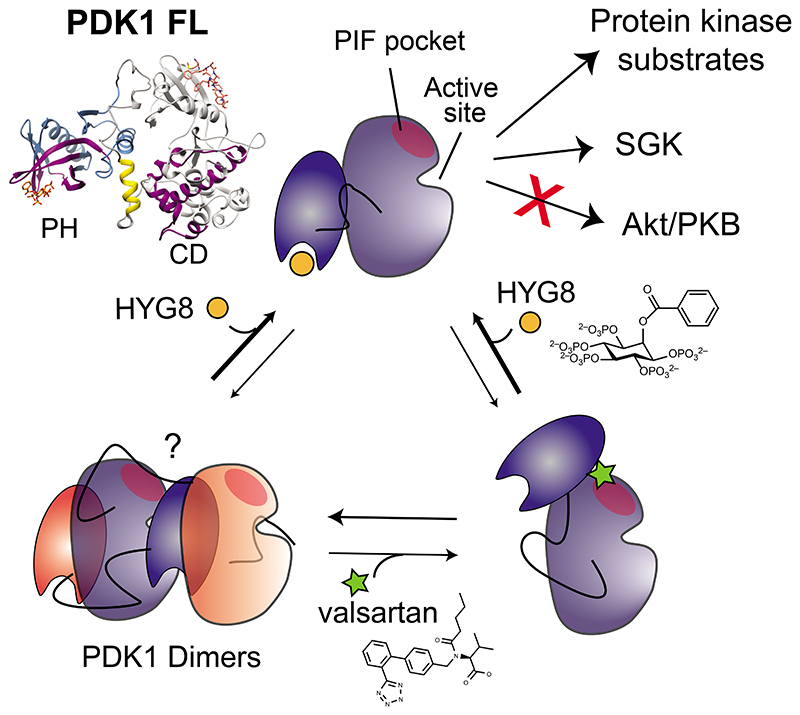
Fig. 8.
Different conformations of full-length PDK1 determine substrate specificity. Small molecules HYG8 and valsartan stabilized different monomeric conformations of full-length PDK1, defined by the relative position of the linker-PH domain. The different conformations of full-length PDK1 had distinct biochemical characteristics. The conformations of PDK1 stabilized by HYG8 protected the “back” of the kinase catalytic domain, had unmodified in vitro activity towards some substrates, such as SGK, but were impaired in the phosphorylation of Akt (also termed PKB) and another particular peptide substrate.