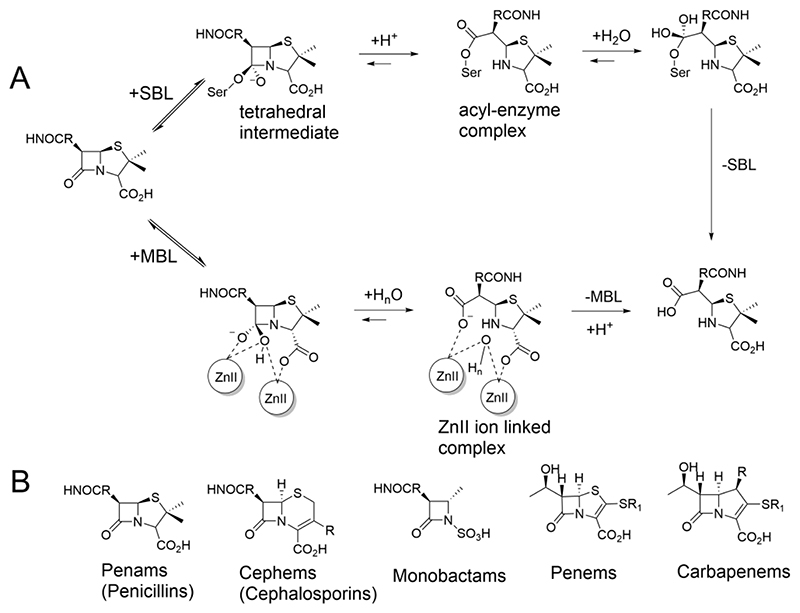
Figure 1. β-Lactam hydrolysis by serine and metallo-β-lactamase.
(A) SBL catalysis proceeds via an acyl-enzyme complex formed after being hydrolysed via a transient tetrahedral intermediate. In MBL catalysis, a zinc ion activated water molecule (that bridges the two Zn ions in the resting states of B1 and B3 MBLs), reacts with the β-lactam ring. Hydrolysis also proceeds via a transient tetrahedral intermediate and a hydrolysed intermediate stabilised through interactions with the Zn ions[6]. (B) Classes of clinically used β-lactam antibiotics.