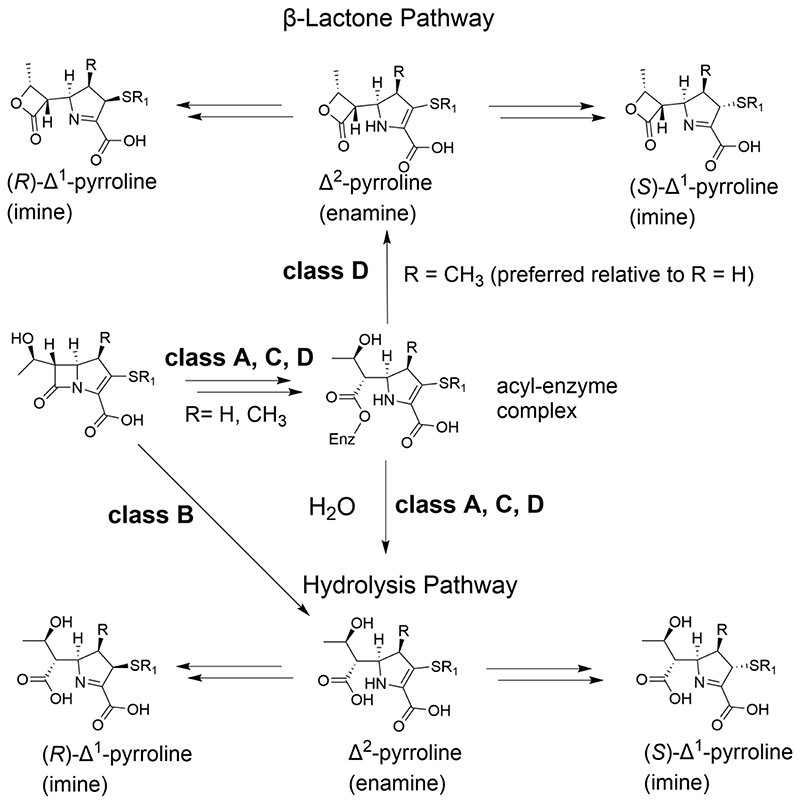
Figure 2. Carbapenem hydrolysis by β-lactamases.
SBL and MBL catalysed carbapenem hydrolysis is proposed to result (predominantly) in the initial formation of an enamine (Δ2-pyrroline) that isomerises to give [(2R)- and (2S)-Δ1-imine] products[10,13]. Various isomeric imine / enamine forms of reacted carbapenems have been observed crystallographically at transpeptidase/SBL/MBL active sites. In the case of the class D SBLs, the acyl-enzyme intermediate can also react to give lactone products.