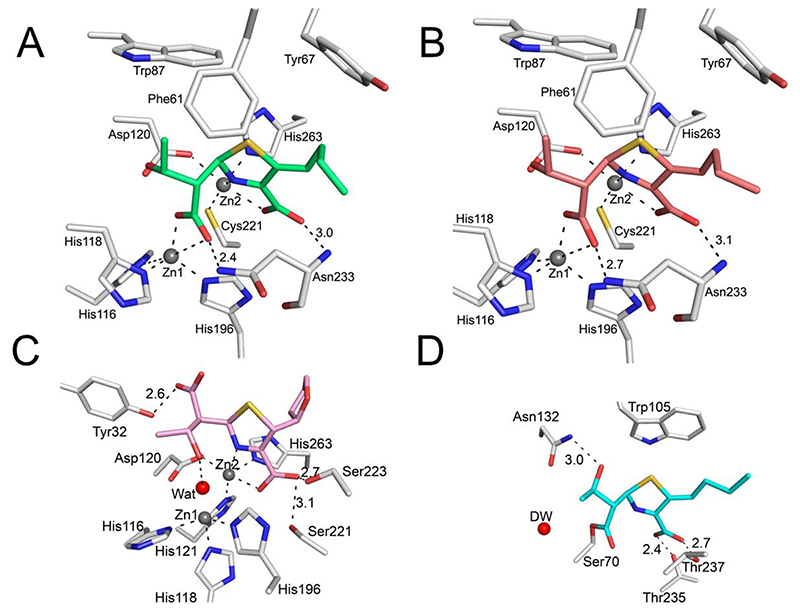
Figure 5. Interactions of the faropenem-derived complexes with serine- and metallo-β-lactamase active sites.
Protein residues are in light grey; faropenem-derived atoms are coloured as in Figure 4. Zinc ions are shown as grey spheres and the catalytic water (Wat) as a red sphere. Metal coordination and hydrogen bonds are shown as black dashes, with the distances labelled in Å. (A) VIM-2 (I222 space group), PDB 7A5Z; (B) VIM-2 (C2 space group) PDB 7A60; (C) L1 PDB 7A63; (D) KPC-2 PDB 7A61. Note the different conformations of the hydroxyethyl group in L1 (C) compared to VIM-2, leading to a lack of interaction with Zn1. KPC-2 (D) forms an acyl-enzyme complex with the faropenem derived complex.