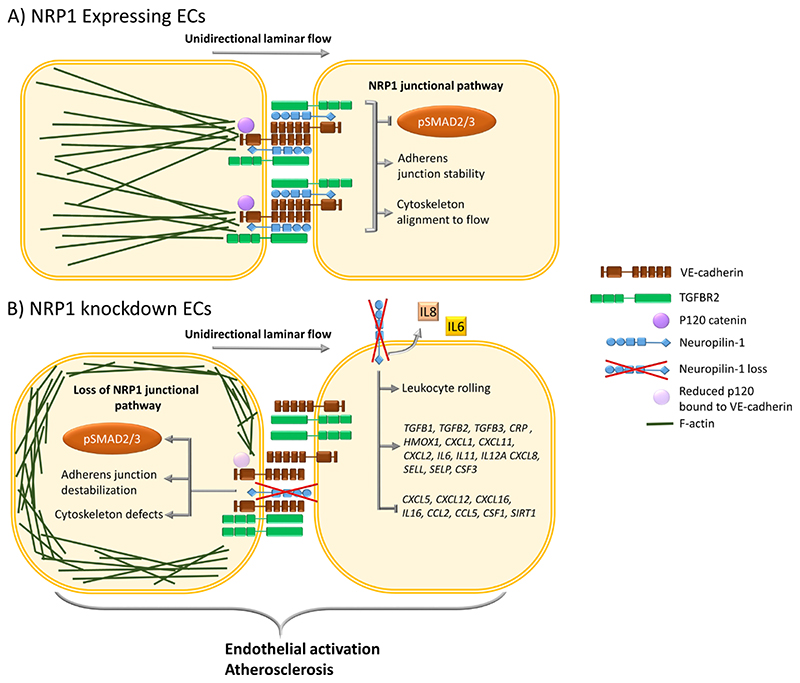
Fig. 6. Schematic representation of the effect of NRP1 on the TGFBR2-VE-cadherin pathway in regulating adherens junctions and inflammatory responses.
(A) In ECs, NRP1 interacts with VE-cadherin and TGFBR2, reducing TGF-β signaling and stabilizing adherens junctions. NRP1 promotes VE-cadherin interaction with p120 catenin, leading to optimal coupling of the actin cytoskeleton with the adherens junction complex and to alignment of the cytoskeleton to the direction of flow. (B) In the absence of NRP1, the interaction of NRP1 with VE-cadherin is lost and that of VE-cadherin with p120 catenin is reduced, leading to cytoskeleton remodeling and abundant cortical actin and resulting in a lack of cytoskeleton alignment to the flow direction. Furthermore, in ECs lacking NRP1, adherens junction destabilization increases the plasma membrane localization of TGFBR2, resulting in the downstream phosphorylation of SMAD2/3. Loss of NRP1-dependent signaling pathways results in EC activation, leading to increases in the expression of genes encoding proinflammatory cytokines, chemokines, adhesion molecules, secretion of IL6 and IL8, leukocyte rolling, and plaque formation in an atherosclerosis mouse model.