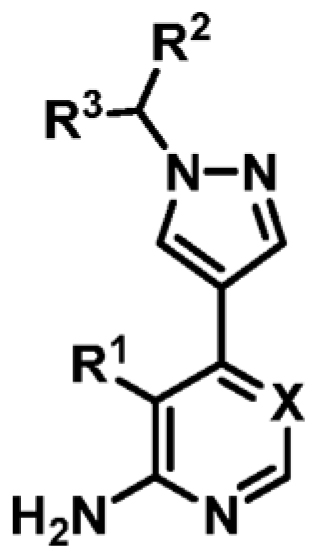
Table 3. Biophysical Data of 4-Amino-6-(pyrazol-4-yl)pyrimidine Derivatives against MabPurCa
 .
.
| Compound | R1 | R2 | R3 | X | ΔTm (°C)b | MabPurC Kd (μM) | LEd |
|---|---|---|---|---|---|---|---|
| 9 | CN | Me | H | N | +4.4 | 23 ± 3 | 0.39 |
| 12 | CN |
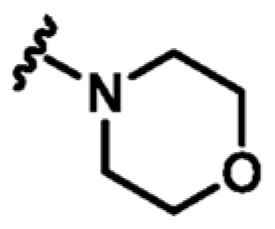
|
=o | N | +2.9 | 45 ± 5 | 0.27 |
| 13 | CN |
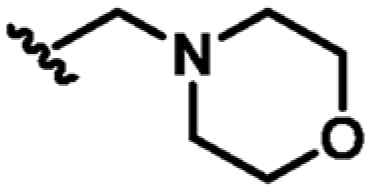
|
H | N | +3.5 | 52 ± 8 | 0.26 |
| 14 | CN |
|
H | N | +4.5 | 22 ± 0 8 | 0.32 |
| 15 | CN |
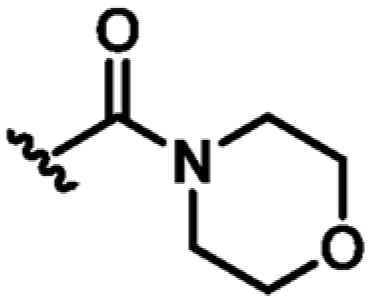
|
H | N | +2.6 | 47 ± 6 | 0.26 |
| 16 | CN | 3-pyridyl | H | N | +6.2 | 3.1 ± 0.3 | 0.36 |
| 17 | CN | 4-pyridyl | H | N | +6.0 | 7.5 ± 0.5 | 0.33 |
| 18 | CN | 5-methoxy-3-pyridyl | H | N | +6.5 | 2.1 ± 0.2 | 0.34 |
| 19 | CN | 3-fluorophenyl | H | N | +8.3 | 0.25 ± 0.03 | 0.41 |
| 20 | CN | 3-(trifluoromethyl)phenyl | H | N | +5.3 | ND | - |
| 21 | CN | 3-fluoro-5-methoxyphenyl | H | N | +8.8a | NDe | - |
| 22 | CN | 4-fluorophenyl | H | N | +9.4 | 0.22 ± 0.02 | 0.41 |
| 23 | CN | phenyl | H | N | +9.3 | 0.28 ± 0.03 | 0.43 |
| 24 | CN | phenyl | Me | N | +7.5 | 0.53 ± 0.03 | 0.38 |
| 25 | CN | benzyl | H | N | +5.3 | ND | - |
| 26 | CN | 3,5-difluorophenyl | H | N | +8.7e | 0.24 ± 0.05 | 0.37 |
| 27 | CN | 3,4-difluorophenyl | H | N | +9.0 | 0.15 ± 0.02 | 0.37 |
| 28 | Cl | 3-fluorophenyl | H | N | +7.1 | 0.35 ± 0.02 | 0.39 |
| 29 | CN | 3-fluorophenyl | H | CH | +5.1 | 1.4 ± 0.06 | 0.36 |
| 30 | Cl | 3-fluorophenyl | H | CH | +2.6c | ND | - |
| 31 | H | 3-fluorophenyl | H | N | +4.1 | ND | - |
ND not determined.
1 mM ligand and 100 μM Mab PurC
0.5 mM ligand and 100 μM Mab PurC
kcal mol–1 per heavy atom.
Due to poor solubility.
