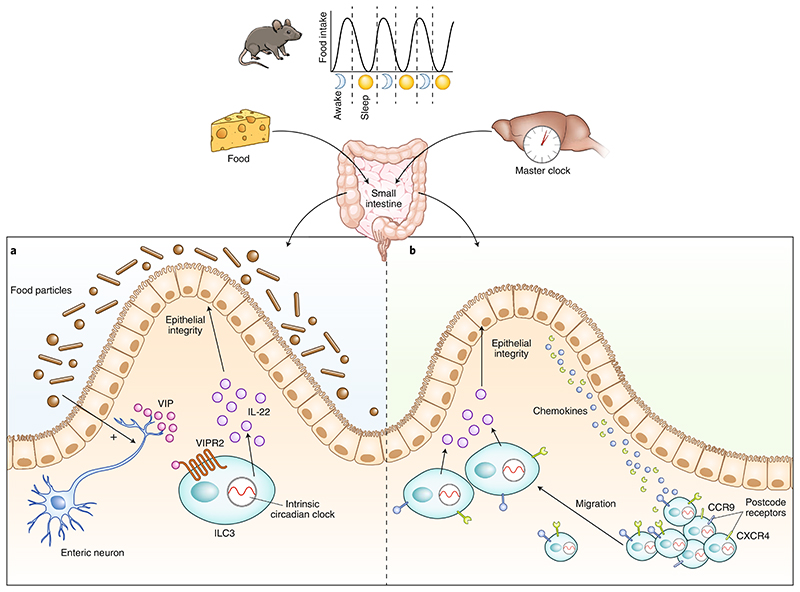
Fig. 1. Neuroimmune regulation of ILC3s in the gut.
ILC3s require a cell-intrinsic circadian clock for their activity. Local and systemic neuronal signaling interacts with the circadian machinery of ILC3s to shape gut homeostasis. a, Seillet et al.1 show that gut ILC3s are located in proximity to enteric neurons at the base of the lamina propria. Food enhances VIP release from enteric neurons. VIP interacts with VIPR2 receptors on the surface of ILC3s to induce the secretion of IL-22, which maintains intestinal epithelial integrity and gut homeostasis. b, Godinho-Silva et al.2 demonstrate that light-entrained signals tuned by the master circadian clock in the brain integrate with the ILC3s’ intrinsic circadian machinery, which in turn promotes the expression of postcode receptors on ILC3s (such as CCR9 or CXCR4) that facilitate chemokine-mediated migration of ILC3s to the base of the intestinal villi to mediate mucosal defense and host homeostasis. ILC3s, type 3 innate lymphoid cells; VIP, vasoactive intestinal polypeptide; VIPR2, vasoactive intestinal polypeptide receptor 2.