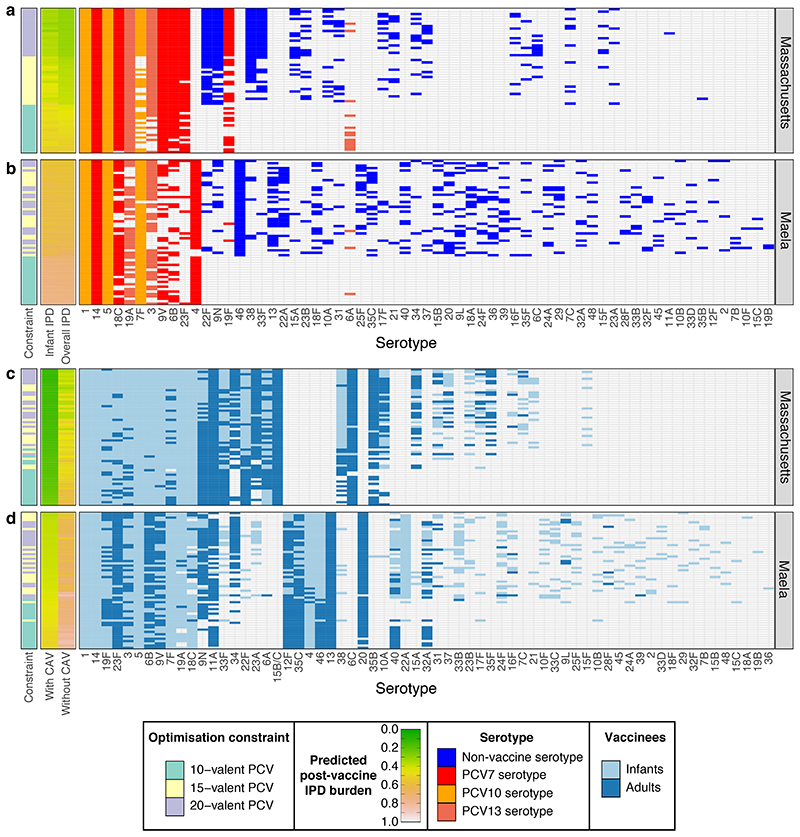
Figure 3. Vaccine strategies for minimising population-wide IPD.
a-b, These plots summarise the infant-administered PCV formulations identified when optimising for minimising both infant and adult IPD under different constraints in a, Massachusetts and b, Maela (n = 20 for each combination of optimisation constraint and criterion in each population). Data are displayed as described in Figure 2c-d, except that the rows are ordered by the predicted post-vaccination overall IPD burden. This assumes herd immunity induced by the infant vaccination campaign would also eliminate the vaccine serotypes from adult IPD. c-d, The plots summarise combined strategies in which complementary adult vaccines (CAVs) were designed for each of the infant vaccinations shown in Figure 2c-d for c, Massachusetts and d, Maela. Pneumococcal population dynamics were assumed to be driven by the carried population in infants, such that elimination of serotypes in infants resulted in population-wide herd immunity. The CAVs were designed to provide protection against the 10 serotypes predicted to cause the most IPD in adults 10 years after the introduction of the infant-administered vaccines. The adult-administered vaccines were assumed not to drive herd immunity themselves. These formulations are represented as in panels a and b, except that the heatmaps show the predicted population-wide IPD burden for infant vaccination without a CAV, or combining infant vaccination with a CAV. The rows are ordered by the overall IPD burden predicted for each combined vaccination strategy, with cells coloured light blue if serotypes were included in the infant-administered formulation, or dark blue if serotypes were included in the adult-administered formulation.