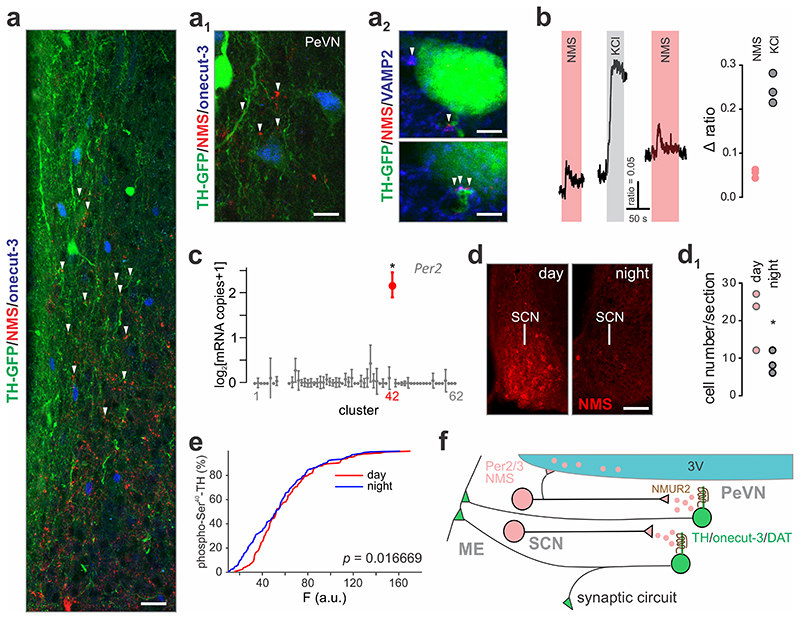
Fig. 8. Periventricular onecut-3+ dopamine neurons respond to neuromedin S produced during light periods.
(a,a1) Immunohistochemical identification of synaptic contacts containing neuromedin S, which innervate onecut-3+ A14 neurons. (a2) VAMP2 was used as a ubiquitous presynaptic marker; perisomatic terminals are shown. (b) Bath application of 500 nM neuromedin S (NMS) leads to the generation of Ca2+ responses in a subset of periventricular TH+ cells, which was comparable to depolarization by 55 mM KCl. (c) The neuronal cluster containing neuromedin S (#42) also expresses the circadian pacemaker gene, Per2. Bold symbol denotes significant expression (> 2x s.e.m. from baseline); *q < 0.05. (d) Circadian fluctuations in neuromedin S content in the suprachiasmatic nucleus (SCN) as detected histochemically. (d1) Quantitative neuromedin S histochemistry using perisomatic fluorescence analysis on SCN neurons (n = 3 animals/group). *p < 0.05. (e) Circadian dependence of tyrosine hydroxylase phosphorylation at Ser40 as revealed by quantitative histochemistry (n = 4 animals/group). Cumulative distribution of function is shown. *p = 0.0167 (two-sample Kolmogorov-Smirnov test). (f) Synaptic wiring of a circadian pacemaker network regulating dopamine release from A14 neurons in the periventricular nucleus of the hypothalamus. Note that both synaptic and volume transmission mechanisms for neuromedin S modulation of dopaminergic output at the median eminence (ME) might exist. Scale bars = 20 μm (a), 10 μm (a1), 5 μm (a2, top), 3 μm (a2, bottom), 50 μm (d).