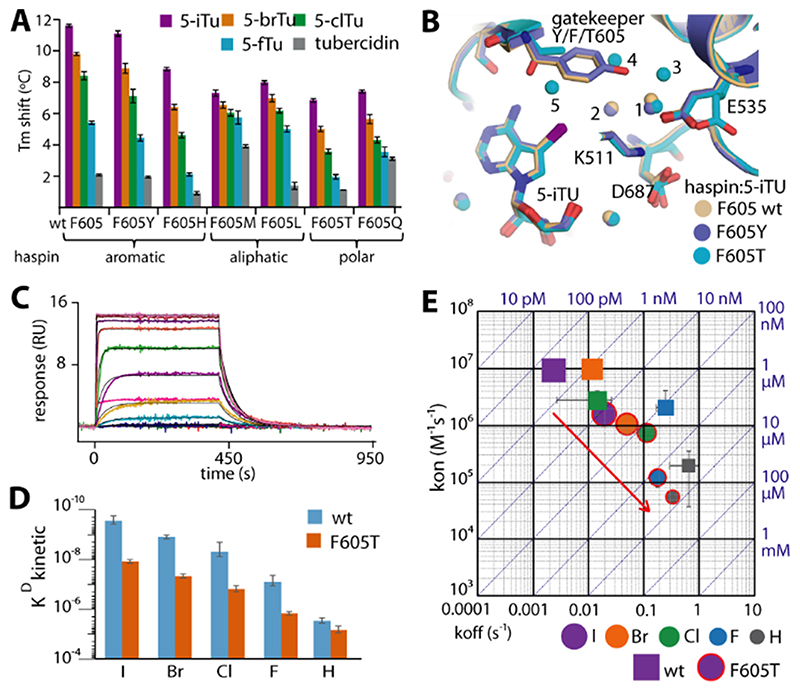
Figure 4. The effect of gatekeeper mutation on the binding kinetics of haspin with tubercidin derivatives.
A) Tm shifts of six haspin mutants against five tubercidin derivatives. B) Superimposition of 5-iTU-complexed crystal structures of wild-type, F605Y, and F605T haspin reveals conserved binding modes for the inhibitor, but differences in the bound water molecules within the binding site. C) The SPR sensorgram demonstrates fast binding kinetics for the interaction between 5-iTU and the F605T mutant, which is accompanied by significant decreases in KD (D) and kon and generally increased koff constants (E).