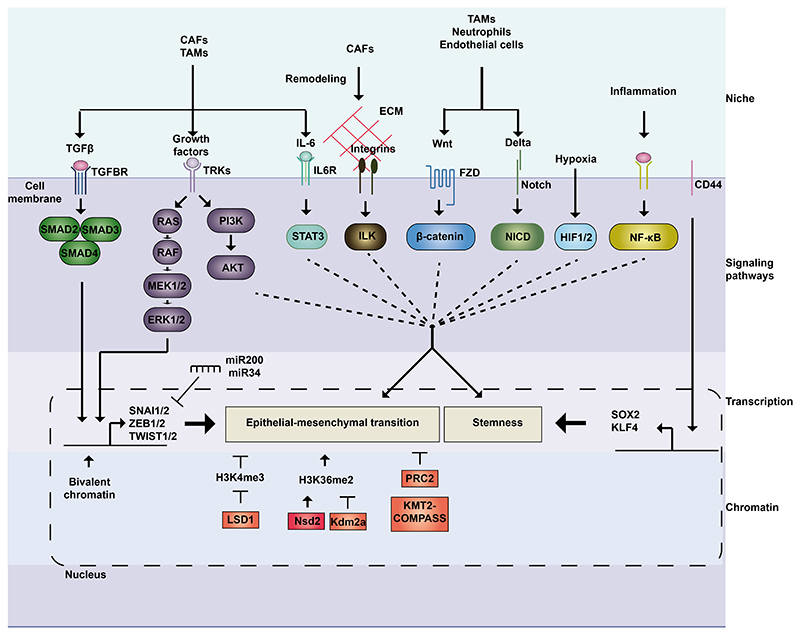
Figure 4. Molecular mechanisms regulating cancer cell plasticity.
Cancer cell plasticity is regulated extracellularly, by signals coming from the microenvironment, and intrinsically, through signaling pathways, transcriptional programs, and chromatin remodeling. TFGβ and RAS-MAPK pathways can act jointly to induce EMT. CD44 and Wnt regulate stemness, while Notch, JAK-STAT and integrins act on stemness and EMT in a context-dependent manner. Hypoxia induces stemness, while NF-κB is involved in plasticity by its role in inflammation. These pathways activate transcriptional programs regulated by key transcription factors involved in EMT (e.g., SNAI1/2, ZEB1/2, TWIST1/2) and stemness (e.g., SOX2, KLF4). Their action can be modulated by negative feedback loops involving miRNAs (e.g., ZEB/miR-200 and SNAI1/- miR-34) and depends on the chromatin landscape. LSD1 can remove the transcriptionally active H3K4me3 histone mark and collaborate with Snai1 to silence epithelial genes. Nsd2 and Kdm2a exhibit antagonist actions, as writer and eraser of H3K36me2, histone mark increased during EMT. PRC2 and KMT2-COMPASS are critical to regulate the epithelial state. CAF, cancer-associated fibroblast; ECM: extracellular matrix; FZD, frizzled; HIF, Hypoxia-inducible factor; IL6R, interleukin-6 receptor; TAM, tumor-associated macrophage; TGFBR, Transforming Growth Factor Receptor; TRK, Tyrosine receptor kinase.