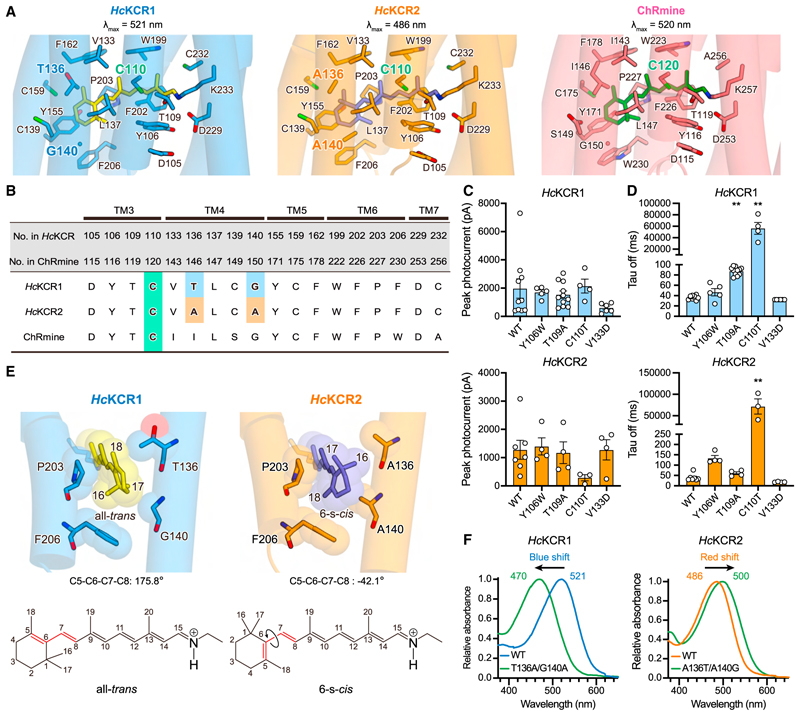
Figure 3. Retinal binding pocket.
(A) Retinal binding pockets of HcKCR1 (left), HcKCR2 (middle), and ChRmine (right) with pocket-forming residues shown in stick models.
(B) Sequence alignment for residues in retinal binding pocket.
(C) Peak photocurrent amplitudes ofWT and four mutants of HcKCR1 (top) and HcKCR2 (bottom), respectively. Mean ± s.e.m. (n = 3–11; Kruskal-Wallistestwith Dunnett’s test).
(D) τoff of WT and four mutants of HcKCR1 (top) and HcKCR2 (bottom), respectively. Mean ± s.e.m. (n = 3–11; Kruskal-Wallis test with Dunnett’s test; **p < 0.01).
(E) β-ionone rings of HcKCR1 and HcKCR2 (top) and chemical structures of all-trans and 6-s-cis-retinal (bottom). Red lines represent C5–C6–C7–C8 bonds.
(F) Absorption spectra of HcKCR1 and 2 WT and their swapping mutants.