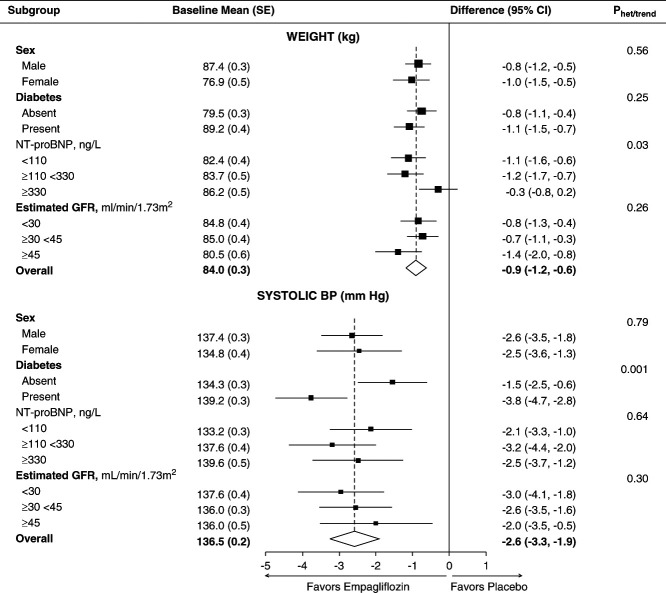
Figure 4.
Full trial cohort: effects of empagliflozin on weight and systolic BP overall and by key bioimpedance substudy prespecified subgroups. Study-average differences are adjusted for baseline values of the dependent variable (in continuous form) and for any differences in key baseline characteristics (categories of age, sex, diabetes, eGFR, urinary albumin-creatinine ratio, and region) between treatment groups and weighted in proportion to the amount of time between follow-up visits (see Supplemental Methods). Each analysis includes all individuals with at least one follow-up measurement of the outcome variable with mean imputation of missing baseline measurements. For comparison, between-group differences in the substudy cohort were −0.7 (95% CI −1.3 to −0.1) kg and −3.3 (−5.5 to −1.2) mm Hg for weight and systolic BP, respectively.