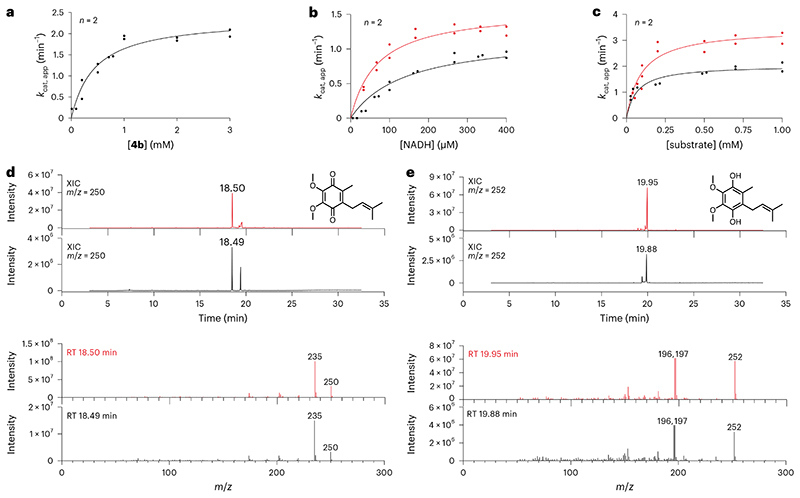
Fig. 5 |. C2 decoration is catalysed by COQ5, COQ7 and COQ3.
a, Michaelis−Menten kinetics of COQ5 in presence of an excess of SAM with 4b as varying substrate. Activity was measured by coupling the enzyme to COQ7 and COQ9 to exploit the NADH consumption spectrofluorimetric assay. b, Michaelis−Menten kinetics of COQ7 in presence of an excess of non-prenylated 5ox with NADH as substrate. Activity was measured in the presence (red) and absence of COQ9 (black). c, Michaelis−Menten curve of the COQ7−COQ9 system in presence of saturating concentration of NADH with 5ox as substrate. Activities were measured using the mono-prenylated (red) and non-prenylated (black) version of the substrate. d, GC/MS analysis of the overnight COQ7−COQ9−COQ3 transformation of 5ox to CoQ1. The XICs and full-scan mass spectra of CoQ1 recorded after the injection of 500 ppm analytical standard and the reaction mixture are shown in red and black, respectively. e, GC/MS analysis of the overnight COQ7−COQ9−COQ3 transformation of 5ox to CoQ1H2. The XICs and full-scan mass spectra of CoQ1H2 recorded after the injection of 500ppm analytical standard and the reaction mixture are shown in red and black, respectively. Individual data points corresponding to n = 2 independent measurements are shown in a−c. RT, retention time.