Fig. 5. Iterative medicinal chemistry furnished an orally bioavailable inhibitor.
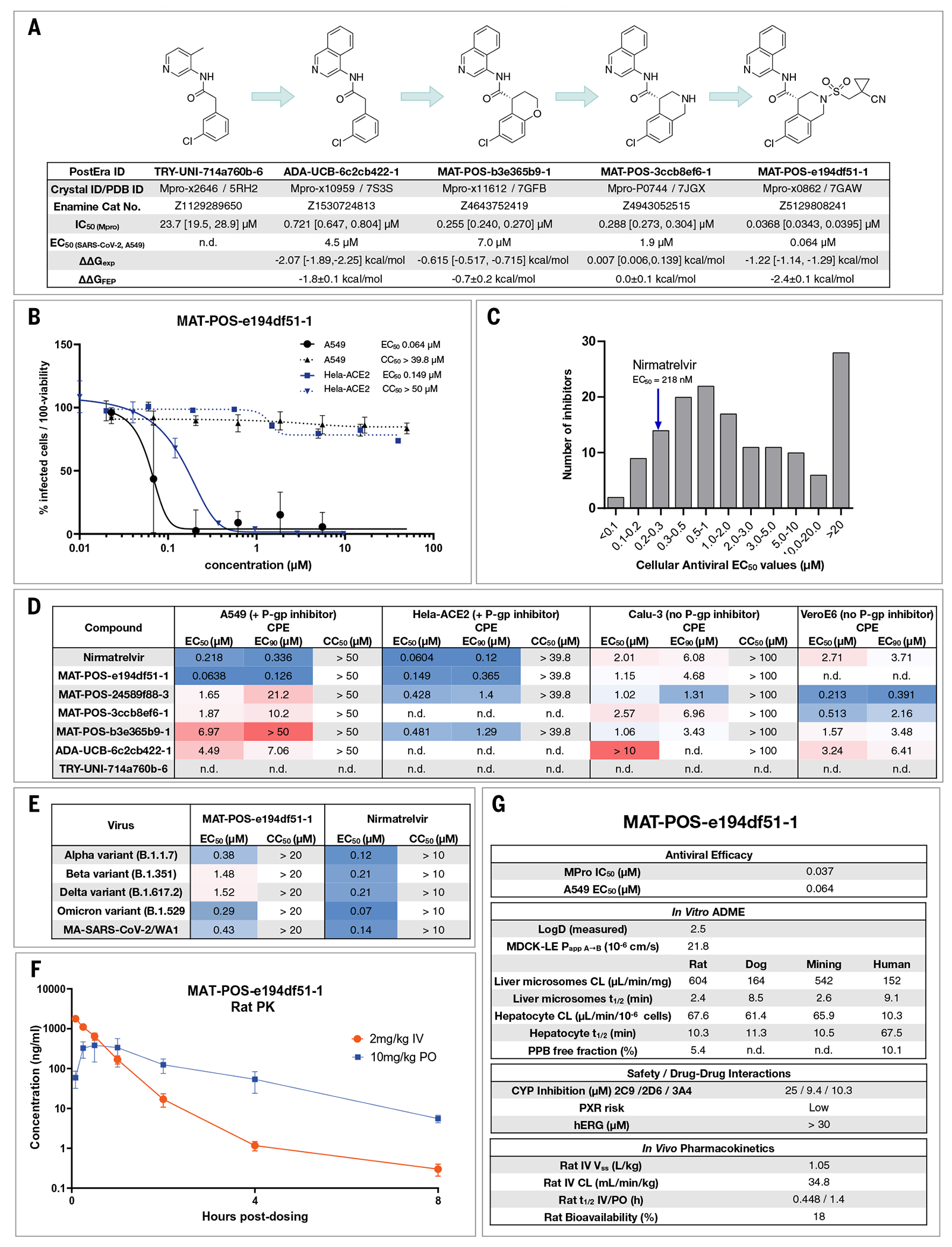
(A) Summary of key medicinal chemistry milestones in developing the initial crowdsourced lead compound into a potent antiviral. X-ray structures for each milestone compound are available via Fragalysis, and each compound can be obtained from Enamine using the corresponding catalog numbers. Retrospective alchemical free-energy calculation predictions for each transformation (ΔΔGFEP) are shown for each step between milestones, along with the corresponding experimental free-energy difference (ΔΔGexp) derived from biochemical activities. As positive control, under our assay condition, nirmatrelvir has an IC50 of 2.6 nM. (B) Antiviral activity of MAT-POS-e194df51-1 cellular antiviral assays, with an EC50 of 64 nM in A549-ACE2-TMPRSS2 cells assessing CPE (black; plotted as 100 – percent viability) and 126 nM in HeLa-ACE2 assays (blue; plotted as percentage of infected cells). Both assays were performed with P-gp inhibitors. (C) Histogram comparing antiviral efficacy of all COVID Moonshot compounds measured to date in an A549-ACE2-TMPRSS2 CPE cellular antiviral assay. (D) Detailed cellular antiviral assessment of key compounds composing the synthetic strategy (A) across different cell lines and assay techniques, with and without p-gp inhibitors, demonstrating efficacy of MAT-POS-e194df51-1 in various setups and laboratories. (E) MAT-POS-e194df51-1 shows good cross-reactivity against known circulating variants of SARS-CoV-2 in antiviral cellular assays in a CPE assay in HeLa-ACE2 cells. (F) PK profile of MAT-POS-e194df51-1 in rats with a 2 mg/kg intravenous and 10 mg/kg oral dosing with good oral availability. (G) ADME characteristics of MAT-POS-e194df51-1 demonstrate a favorable safety profile, indicating translational potential of the lead series.
