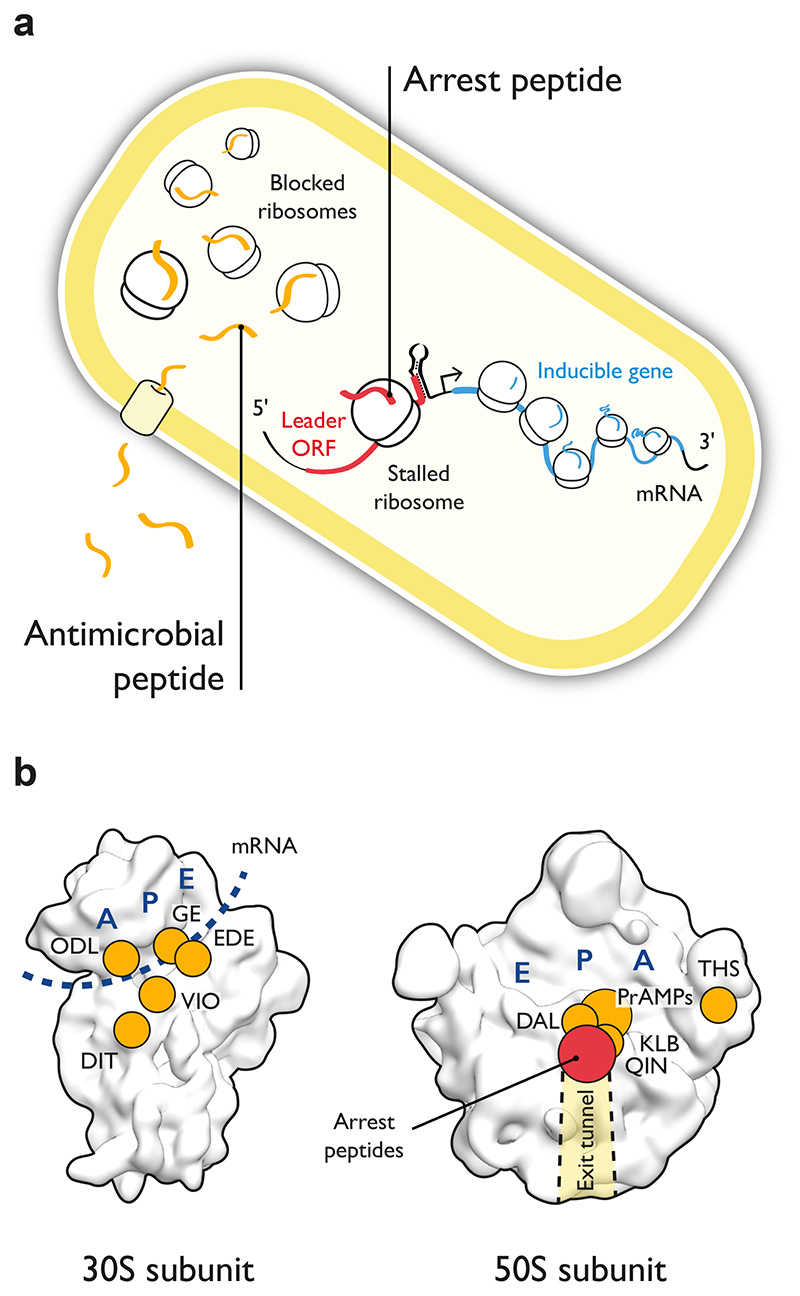
Figure 1. Antimicrobial peptides and arrest peptides are trans- and cis-acting inhibitors of bacterial translation, respectively.
(a) Antimicrobials peptides (left part, orange) are produced by the host immune response of eukaryotes and must cross the bacterial membrane to inhibit the translational machinery in trans. Arrest peptides (center, red) are cis-acting inhibitors of the translational machinery that regulate the expression of inducible genes in bacteria and in eukaryotes. Inhibition in cis results from interactions between an arrest peptide in its nascent state and components of the large ribosomal subunit. (b) Surface representation of the E. coli 30S and 50S ribosomal subunits (PDB: 4ybb 96) showing the sites of action of various ribosome-targeting antimicrobial peptides (orange) and of arrest peptides (red). Abbreviations for antimicrobial peptides are DAL (Dalfopristin), DIT (Dityromycin), EDE (Edeine), GE (GE81112), KLB (Klebsazolicin), ODL (Odilorhabdin), PrAMPs (Proline-rich antimicrobial peptides), QIN (Quinupristin), THS (Thiostrepton), VIO (Viomycin). The A, P and E tRNA binding sites are in dark blue and the path of the mRNA on the 30S subunit is indicated with a dark blue dotted line. The nascent polypeptide exit tunnel is indicated with a shaded area.