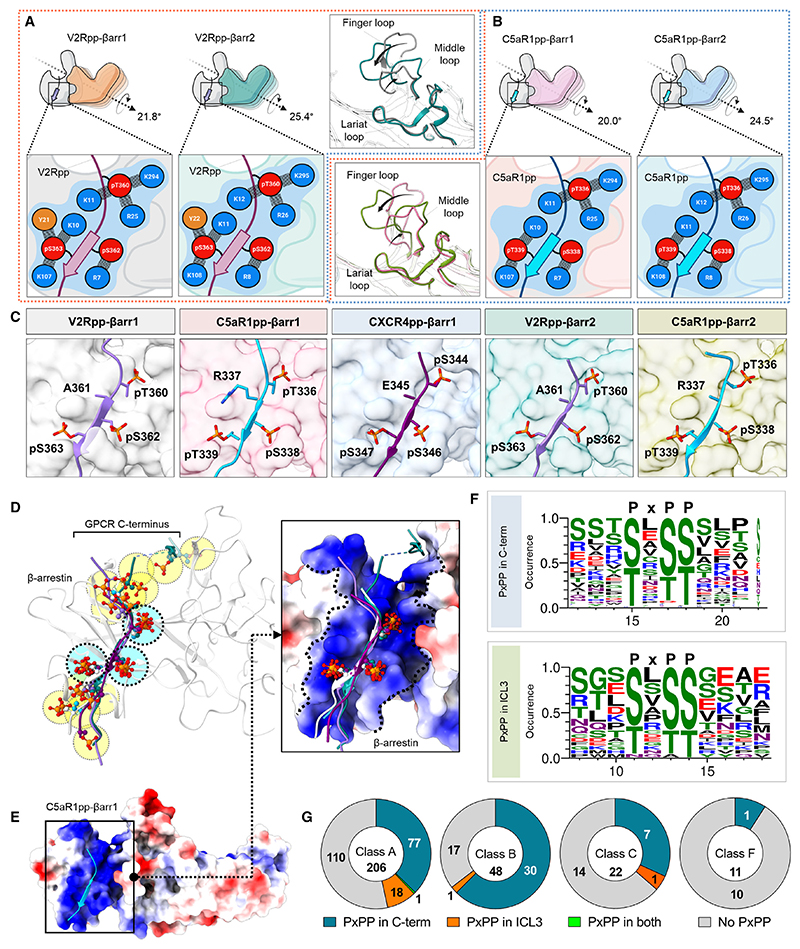
Figure 6. Identification of a key phosphorylation motif in GPCRs driving βarr activation.
(A) Comparison of the V2Rpp-bound βarr1 and βarr2 structures reveals similar interactions of V2Rpp with both isoforms of βarrs although a slightly higher inter-domain rotation is observed in βarr2 (left). A schematic representation of the interface network between negatively charged phospho-residues (red) and positively charged residues (blue) of βarrs is shown (below, zoomed-in box). Although the lariat loops of the two structures align well, significant deviations can be observed for the finger and middle loops (right, inset box).
(B) Comparative analysis of C5aR1pp-bound βarr1 and βarr2 structures uncover similar interactions of C5aR1pp with both βarr isoforms, but again, a higher inter-domain rotation is observed for βarr2. A similar representation of the interface between negatively charged phospho-residues (red) and positively charged residues (blue) of βarrs is shown (below, zoomed-in box).
(C) In all the structures of phosphopeptide-bound βarrs, a conserved motif can be observed with respect to three phospho-residues, referred to as the P-X-P-P motif, where “P” is a phospho-Ser/Thr and “X” can be any other residue.
(D) Superposition of V2Rpp-βarr1 (PDB 4JQI), V2Rpp-βarr2, C5aR1pp-βarr1, CXCR4pp-βarr1, and C5aR1pp-βarr2 shows conservation of phosphates corresponding to P-X-P-P position, whereas other phosphates are distributed throughout the phosphopeptides.
(E) Superposition of phosphopeptides on C5aR1pp-βarr1 reveals the conserved phospho-residues on positively charged cleft present on βarrs’ N-domain. βarr is shown as Coulombic-charged surface here.
(F) A sequence alignment of the C-terminal tail and ICL3 residues of non-olfactory and non-orphan class A receptors reveal the consensus sequence, “P-X-P-P” required for activation of βarrs. The consensus sequence logo was generated with the WEBLOGO tool48 and sequence alignment was performed with Kalign.49 A stretch of 11 amino acid residues has been shown for better representation.
(G) Proportions of GPCRs of class A, B, C, and F having P-X-P-P motif in C terminus or ICL3 have been represented as pie charts. See also Figure S6 and Table S1.