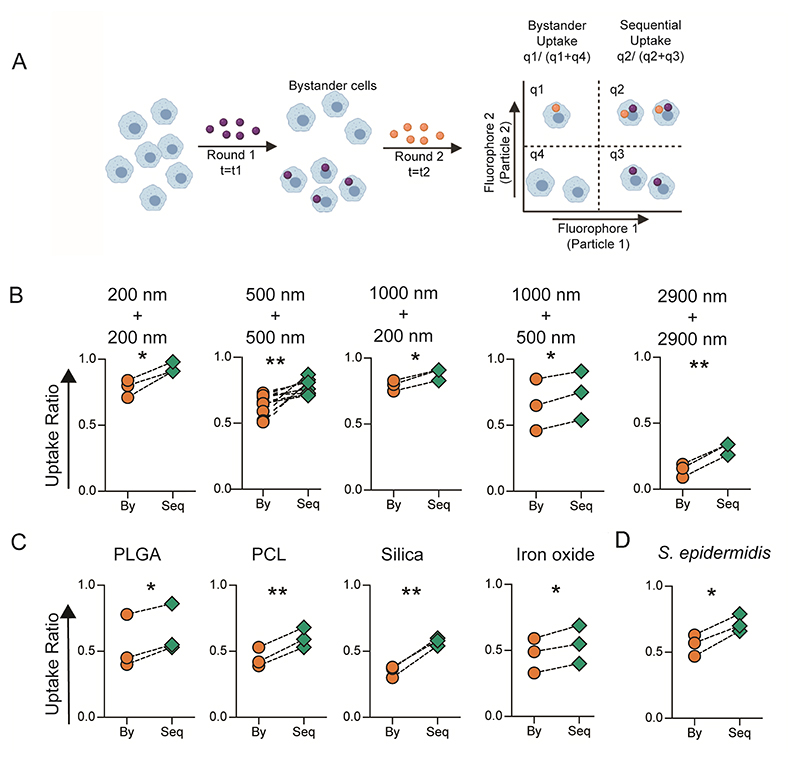
Figure 1. In Vitro Sequential Phagocytosis.
A − Schematic describing the experimental design for calculating bystander and sequential uptake. B − Comparison of bystander (By) and sequential (Seq) uptake of carboxylated polystyrene (PS) particles of different diameters by RAW macrophages. In these experiments, t1 and t2 are 2 hours each, and cell to particle ratio was 1:250 for 200 nm, 1:50 for 500 nm, 1:50 for 1000 nm and 1:2 for 2900 nm PS particles. C − Sequential uptake with 2-3 μm sized particles composed of PLGA (5 μg/ml), PCL (5 μg/ml), Silica (1:20) and Iron oxide (15μg/ml) (C), added to RAW macrophages in the first round followed by incubation with 1000 nm (1:10) or 500 nm (1:50) carboxylated PS particles in the second round of phagocytosis; PLGA = poly (lactic-co-glycolic) acid; PCL = polycaprolactone. D − Sequential uptake with bacteria, S. epidermidis, added to RAW macrophages in the first round followed by incubation with 500 nm (1:50) carboxylated PS particles in the second round of phagocytosis. Uptake percentages were quantified using flow cytometry. Data sets are representative of n ≥ 3 (independent experiments). * = p < 0.05 and ** = p < 0.01 calculated using paired Student’s t-test.